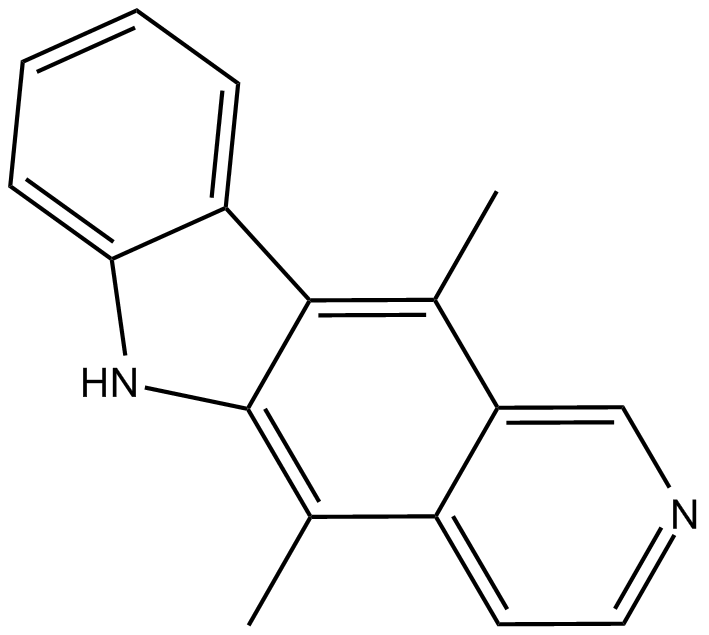
Ellipticine
IC50 = 0.99 μM for L1210 lymphocytic leukemia cells [1]
Plant alkaloid ellipticine shows antitumor, mutagenic and cytotoxic activities by inhibition of DNA topoisomerase II activity. DNA topoisomerase II regulates the overwinding or underwinding of DNA by cuting DNA double helix, passing another unbroken DNA helix through it, and then reannealing the cut strands.
In vitro: Treatment mammalian DNA topoisomerase II reaction mixture with ellipticine resulted in the stimulation of DNA cleavage. The drug-stimulation of DNA cleavage is related to the formation of a ternary complex between topoisomerase II, DNA, and ellipticine. Ellipticine dose not inhibit enzyme-mediated DNA religation, however, it stimulates DNA breakage by enhancing the forward rate of cleavage [2]. Ellipticine showed growth inhibition activity on L1210 lymphocytic leukemia cells with a IC50 of 0.99 μM [1].
In vivo: Ellipticine was evaluated in P. berghei infected mice in the 4-day suppressive test. Ellipticine had a 100% inhibition versus controls on days 5 and 7 at an oral dose of 50 mg/kg/day, and the mean survival time (MST) was more than 40 days [3].
Clinical trial: Several ellipticine derivatives have been validated in clinical trials, however, due to adverse side-effects, no progress has be reported.
References:
[1] Paoletti C, Cros S, Xuong ND, Lecointe P, Moisand A. Comparative cytotoxic and antitumoral effects of ellipticine derivatives on mouse L 1210 leukemia. Chem Biol Interact. 1979 Apr;25(1):45-58.
[2] Tewey KM, Chen GL, Nelson EM, Liu LF. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182-7.
[3] Rocha e Silva LF, Montoia A, Amorim RC, Melo MR, Henrique MC, Nunomura SM, Costa MR, Andrade Neto VF, Costa DS, Dantas G, Lavrado J, Moreira R, Paulo A, Pinto AC, Tadei WP, Zacardi RS, Eberlin MN, Pohlit AM. Comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. Phytomedicine. 2012 Dec 15;20(1):71-6.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 246.31 |
| Cas No. | 519-23-3 |
| Formula | C17H14N2 |
| Solubility | ≥24.6 mg/mL in DMSO with gentle warming; insoluble in EtOH; insoluble in H2O |
| Chemical Name | 5,11-dimethyl-6H-pyrido[4,3-b]carbazole |
| SDF | Download SDF |
| Canonical SMILES | CC1=C2C(NC3=CC=CC=C23)=C(C)C4=CC=NC=C14 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure