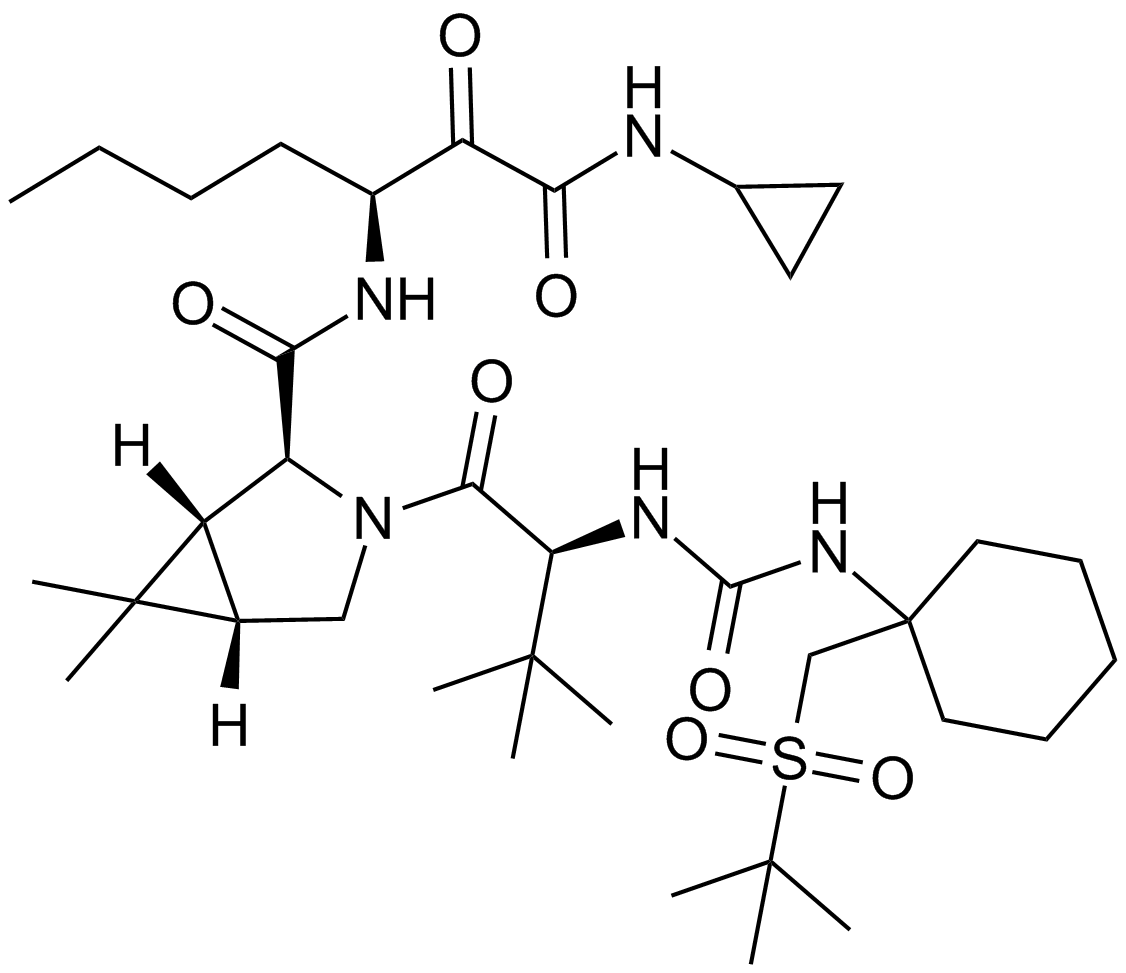
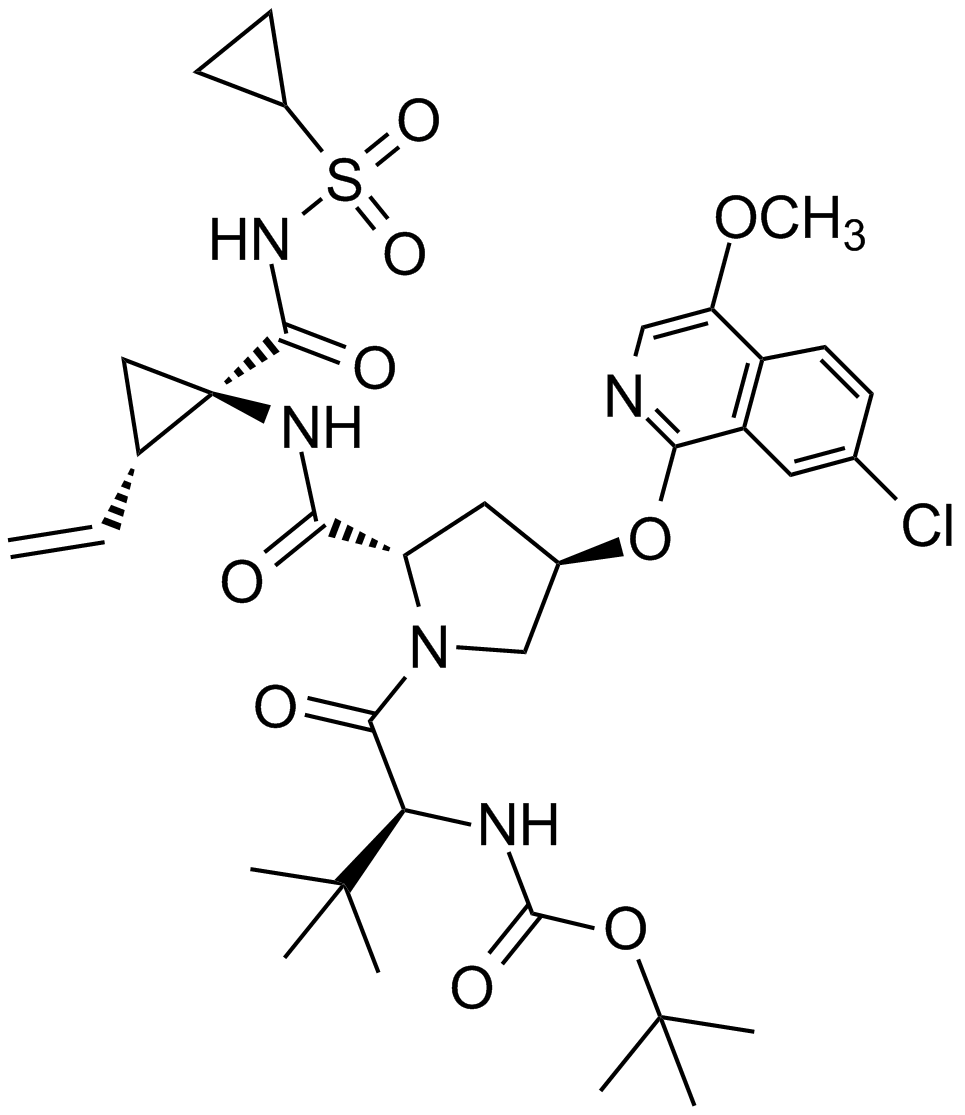
Danoprevir (RG7227)
Danoprevir (R7227) is a potent and selective inhibitor of Hepatitis C Virus (HCV) NS3/4A protease, a chymotrypsin-like serine protease playing an essential role in the viral replication process of HCV, that non-covalently binds to and hence inhibits HCV NS3 protease with 50% inhibition concentration IC50 values ranging from 0.2 to 3.5 nM. X-ray crystallographic analysis has revealed that the cyclopropyl acylsulfonamide of danoprevir occupies the SI/SI’ pocket of HCV NS3 protease with the acyl carbonyl oxygen forming hydrogen bonds to Gly137 and Ser138 in the oxyanion hole of the protease active site and the acyl sulfonamide nitrogen forming a hydrogen bond with His57.
Reference
Jiang Y, Andrews SW, Condroski KR, Buckman B, Serebryany V, Wenglowsky S, Kennedy AL, Madduru MR, Wang B, Lyon M, Doherty GA, Woodard BT, Lemieux C, Do MG, Zhang H, Ballard J, Vigers G, Brandhuber BJ, Stengel P, Josey JA, Beigelman L, Blatt L, Seiwert SD. Discovery of Danoprevir (ITMN-191/R7227), a Highly Selective and Potent Inhibitor of Hepatitis C Virus (HCV) NS3/4A Protease. J Med Chem. 2013 May 28. [Epub ahead of print]
- 1. Jihwan Yu, Juae Jin, et al. "Programmable RNA acetylation with CRISPR–Cas13." Nat Chem Biol. 2025 Jun 2 PMID: 40456962
- 2. Unyime M Effiong, Hannah Khairandish, et al. "Turn-on protein switches for controlling actin binding in cells." bioRxiv. 2023 Oct 26:2023.10.26.561921 PMID: 38992021
- 3. Hui-Shan Li, Nicole M Wong, et al. "High-performance multiplex drug-gated CAR circuits." Cancer Cell. 2022 Nov 14;40(11):1294-1305.e4. PMID: 36084652
- 4. Jason D Vevea, Edwin R Chapman. "Acute disruption of the synaptic vesicle membrane protein synaptotagmin 1 using knockoff in mouse hippocampal neurons." Elife. 2020;9:e56469. PMID: 32515733
- 5. Foight GW, Wang Z, et al. "Multi-input chemical control of protein dimerization for programming graded cellular responses." Nat Biotechnol. 2019 Sep 9. PMID: 31501561
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 731.83 |
| Cas No. | 850876-88-9 |
| Formula | C35H46FN5O9S |
| Synonyms | Danoprevir, RG7227, ITMN-191 |
| Solubility | ≥32.6 mg/mL in DMSO; insoluble in H2O; ≥46.4 mg/mL in EtOH with ultrasonic |
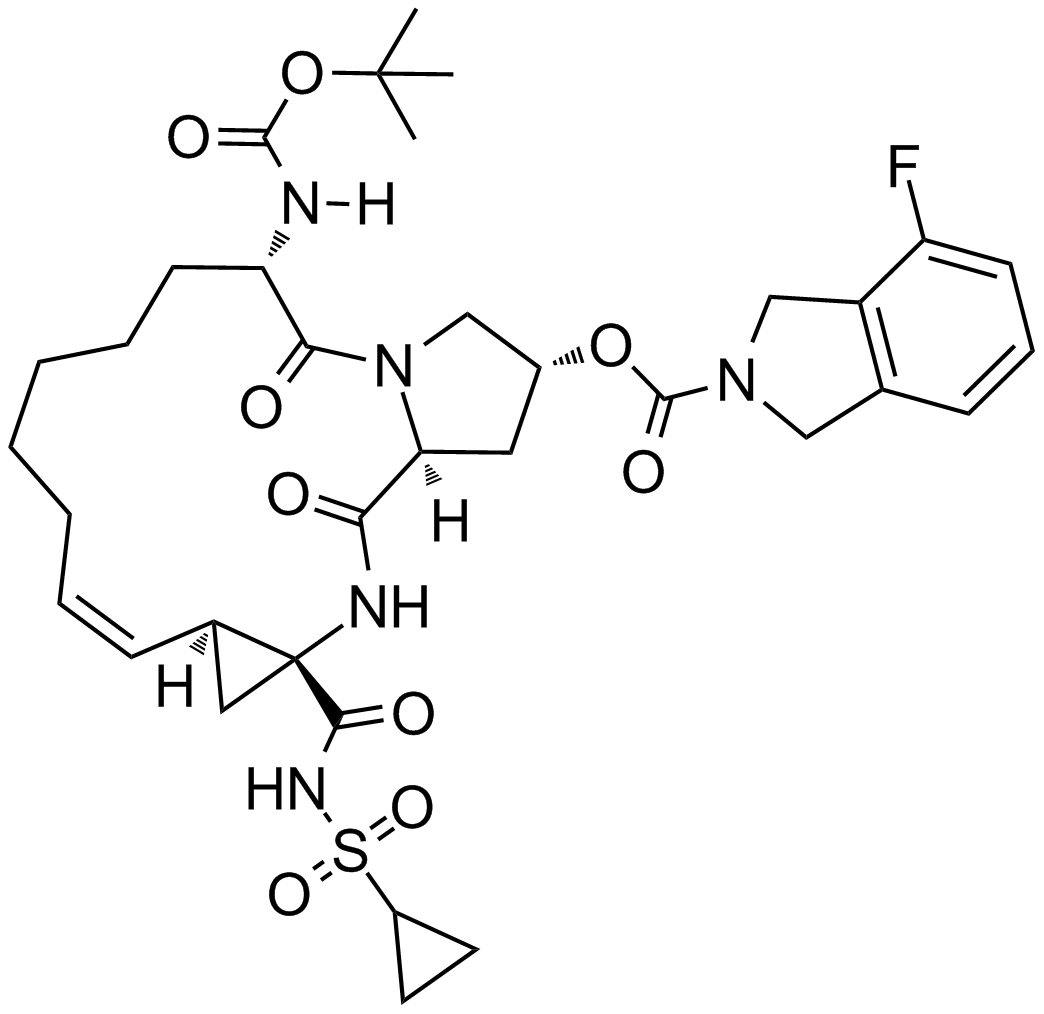
| Chemical Name | (2R,6S,13aS,14aR,16aS,Z)-6-((tert-butoxycarbonyl)amino)-14a-((cyclopropylsulfonyl)carbamoyl)-5,16-dioxo-1,2,3,5,6,7,8,9,10,11,13a,14,14a,15,16,16a-hexadecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecin-2-yl 4-fluoroisoindoline-2-carboxylate |
| SDF | Download SDF |
| Canonical SMILES | FC1=CC=CC2=C1CN(C2)C(O[C@@H]3C[C@](C(N[C@@]4(C(NS(=O)(C5CC5)=O)=O)[C@@](/C=C\CCCCC[C@@H]6N([H])C(OC(C)(C)C)=O)([H])C4)=O)([H])N(C6=O)C3)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Binding assays |
Protease activity for K2040 and genotype 1 to 6 NS3 proteins was followed in a continuous fluorescent resonance energy transfer (FRET)-based assay. The assay buffer contained 25 μM NS4A peptide, 50 mM Tris-HCl, pH 7.5, 15% (vol/vol) glycerol, 0.6 mM lauryldimethylamine N-oxide, 10 mM dithiothreitol, and 0.5 μM fluorescein/QXL520-labeled FRET substrate. Typically, 50 pM K2040 enzyme was added to initiate the reaction. Reactions were set up in black 96-well plates, and fluorescence data were collected using a SpectraMax M5 plate reader. Recovery of activity from preformed ITMN-191·NS3/4A complex was assessed by preincubating 10 nM NS3/4A with a twofold excess of ITMN-191 in 1× assay buffer for 15 min, followed by a rapid 200-fold dilution of the preformed complex into assay buffer containing substrate. |
| Cell experiment [1]: | |
|
Cell lines |
Huh7 cells harboring HCV replicon |
|
Preparation method |
The solubility of this compound in DMSO is >32.6mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
antiviral assays: 100 nM to 5 pMcytotoxicity assays: 1 mM to 5.6 nM |
|
Applications |
ITMN-191 displayed a high degree of specificity for its intended target. In replicon-bearing cells, ITMN-191 (3.7 nM-15 nM) promoted a 3.7 log10 reduction in replicon levels upon 14 days of in vitro treatment but did not clear HCV replicon from every cell. Treatment with ITMN-191 (45 nM) reduced HCV replicon RNA levels and completely cleared replicon RNA. |
| Animal experiment [1]: | |
|
Animal models |
Rats and monkeys |
|
Dosage form |
Oral gavage, 30 mg/kg |
|
Application |
Danoprevir (30 mg/kg) administered to rats or monkeys shows that its concentrations in liver 12 hours after dosing exceed the Danoprevir concentration required to eliminate replicon RNA from cells. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Seiwert S D, Andrews S W, Jiang Y, et al. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227)[J]. Antimicrobial agents and chemotherapy, 2008, 52(12): 4432-4441. |
|
| Description | Danoprevir is a highly selective and potent inhibitor of HCV NS3/4A protease with IC50 value of 0.2-3.5 nM. | |||||
| Targets | HCV NS3/4A protease | |||||
| IC50 | 0.2-3.5 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data