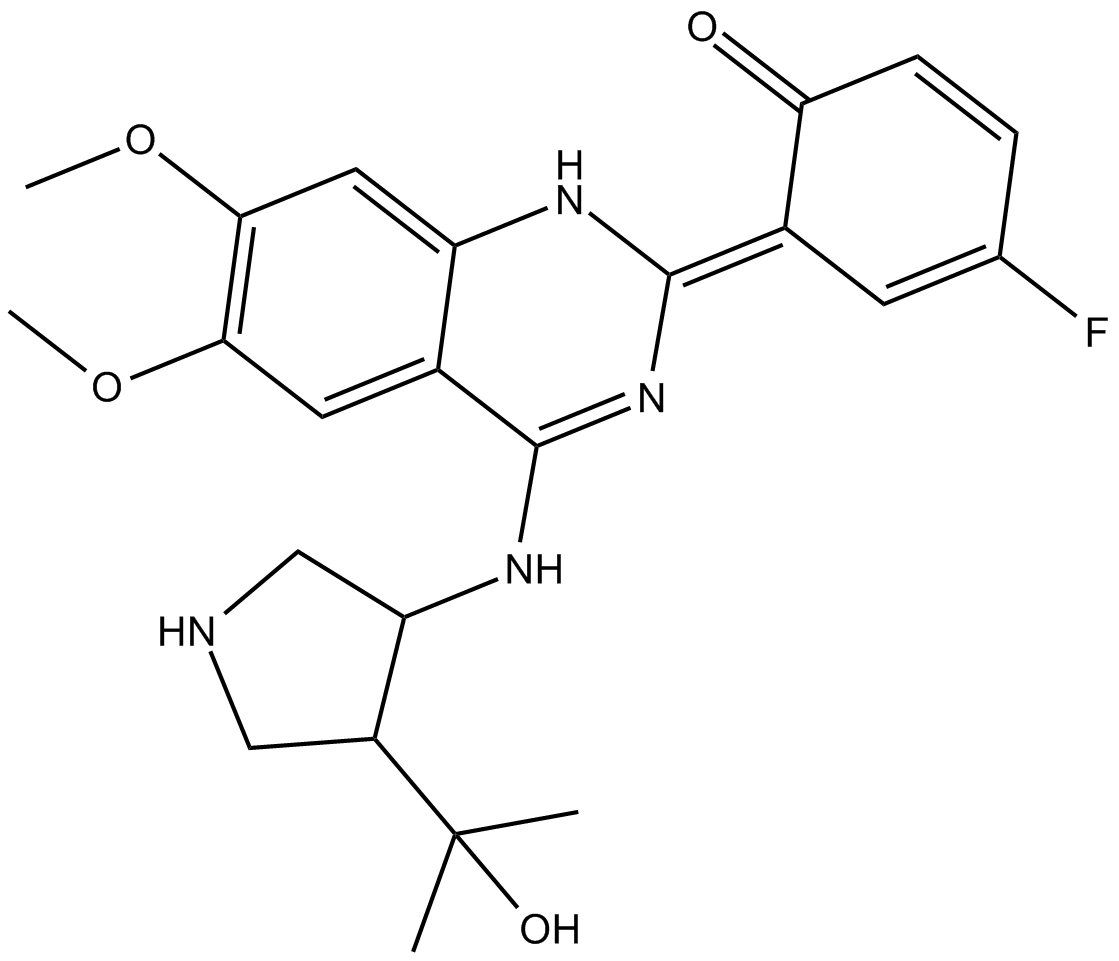
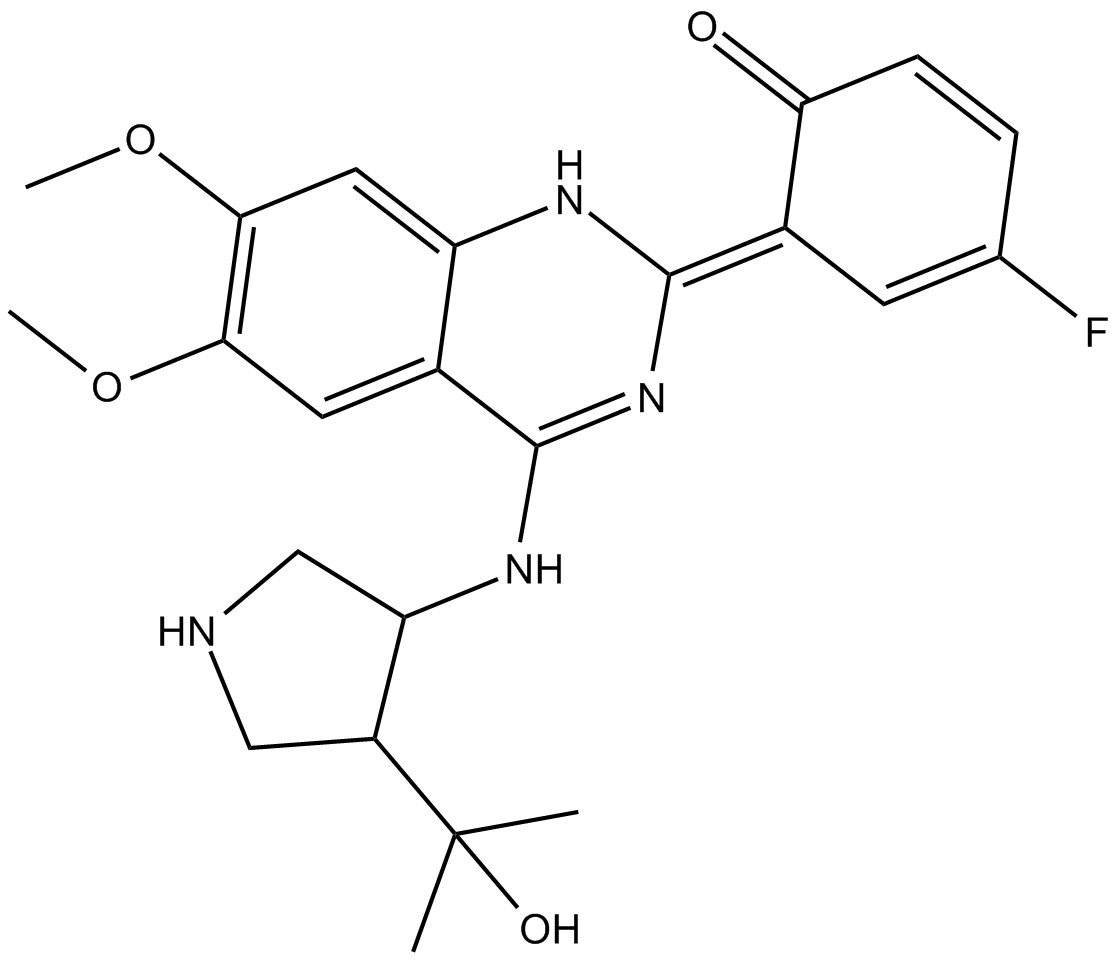
CCT241533
CHK2 is a checkpoint kinase involved in the ATM-mediated response to double-strand DNA breaks. Inhibitors of CHK2 may increase the efficacy of genotoxic cancer therapies. CCT241533 has been identified and characterized as a novel CHK2 kinase inhibitor.
In vitro: CCT241533 inhibits CHK2 with an IC50 of 3 nmol/L and shows minimal cross-reactivity against a panel of kinases at 1 mmol/L. CCT241533 did not potentiate the cytotoxicity of a selection of genotoxic agents in several cell lines [1]. Moreover, as the most potent CHK2 inhibitor identified in the series, CCT241533 shows potent selectivity (63-fold) over CHK1 and low hERG inhibition (hERGIC50=22 μM) [2].
In silico: X-ray crystallography confirmed that CCT241533 bound to CHK2 in the ATP pocket. Overall, the binding mode was found to be very highly conserved relative to previous compounds, with all of the key hydrogen bond interactions maintained. The potency gained with CCT241533 therefore appears to be due to the presence of the two methoxy substituents occupying the solvent exposed region of the enzyme, and contributions from the isopropyl alcohol substituent, which may participate in a second intramolecular hydrogen bond to the quinazoline exocyclicNH [2].
Clinical trial: No clinical data are available.
References:
[1] Anderson VE, Walton MI, Eve PD, Boxall KJ, Antoni L, Caldwell JJ, Aherne W, Pearl LH, Oliver AW, Collins I, Garrett MD. CCT241533 is a potent and selective inhibitor of CHK2 that potentiates the cytotoxicity of PARP inhibitors. Cancer Res. 2011;71(2):463-72.
[2] Caldwell JJ, Welsh EJ, Matijssen C, Anderson VE, Antoni L, Boxall K, Urban F, Hayes A, Raynaud FI, Rigoreau LJ, Raynham T, Aherne GW, Pearl LH, Oliver AW, Garrett MD, Collins I. Structure-based design of potent and selective 2-(quinazolin-2-yl)phenol inhibitors of checkpoint kinase 2. J Med Chem. 2011;54(2):580-90.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 442.48 |
| Cas No. | 1262849-73-9 |
| Formula | C23H27FN4O4 |
| Synonyms | CCT 241533;CCT-241533 |
| Solubility | Soluble in DMSO |
| Chemical Name | (E)-4-fluoro-6-(4-((4-(2-hydroxypropan-2-yl)pyrrolidin-3-yl)amino)-6,7-dimethoxyquinazolin-2(1H)-ylidene)cyclohexa-2,4-dienone |
| SDF | Download SDF |
| Canonical SMILES | CC(C1CNCC1NC2=N/C(NC3=CC(OC)=C(OC)C=C32)=C4C=C(F)C=CC\4=O)(O)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
HeLa and HT-29 cells |
|
Preparation method |
Soluble in DMSO. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
2.2 μmol/L for HeLa cells, 1.7 or 3 μmol/L for HT-29 cells; 24 hrs |
|
Applications |
In the presence of CCT241533, HeLa (2.2 μmol/L CCT241533) and HT-29 cells (1.7 μmol/L CCT241533) exhibited enhanced sensitivity to AG14447. In HeLa cells, the combination of CCT241533 (3 μmol/L) and Olaparib showed significant potentiation. |
|
References: [1]. Anderson VE, Walton MI, Eve PD, Boxall KJ, Antoni L, Caldwell JJ, Aherne W, Pearl LH, Oliver AW, Collins I, Garrett MD. CCT241533 is a potent and selective inhibitor of CHK2 that potentiates the cytotoxicity of PARP inhibitors. Cancer Res. 2011;71(2):463-72. | |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure