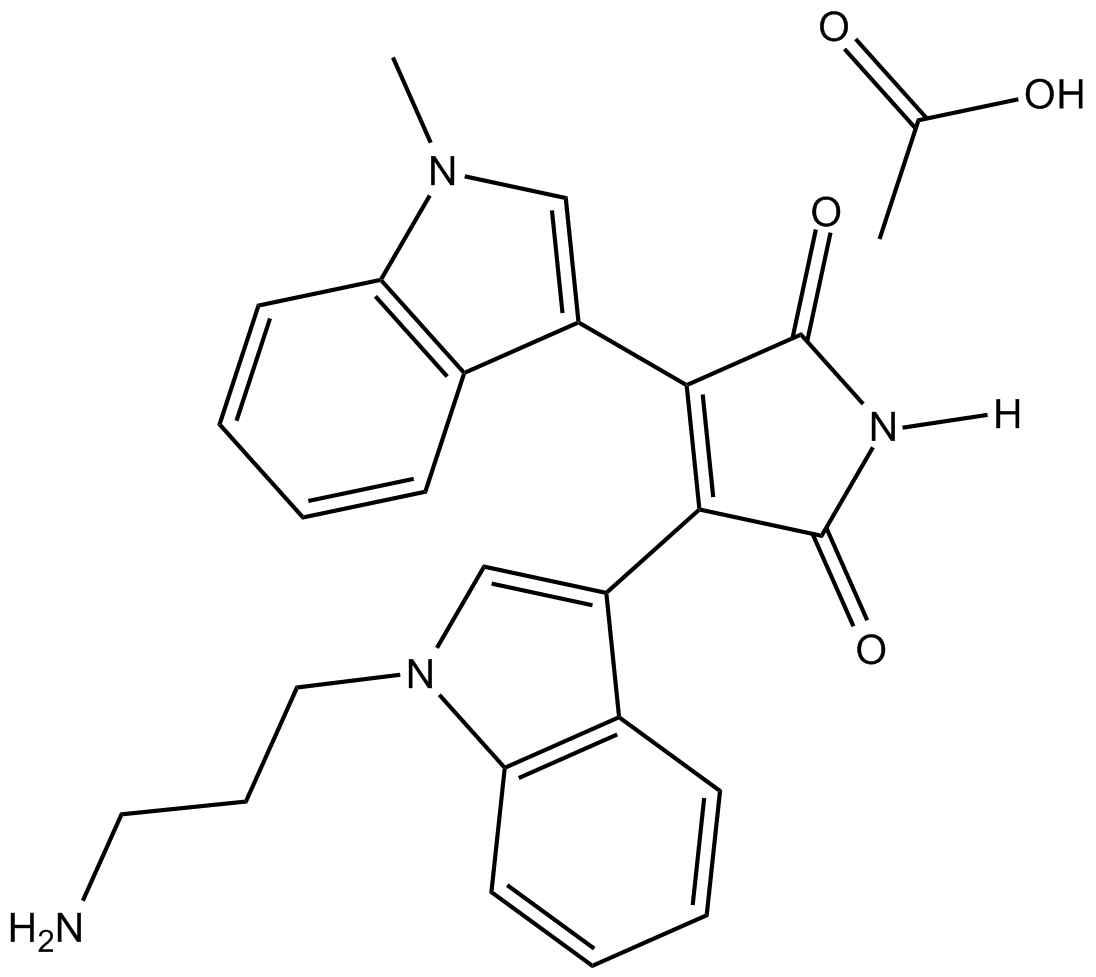
Bisindolylmaleimide VIII (acetate)
IC50: 158 nM for rat brain PKC
Bisindolylmaleimide VIII is a protein kinase C (PKC) inhibitor.
The protein kinase C (PKC) consists of a family of closely related isoenzymes mediating various signal transduction processes. The ten isoenzymes that have been currently identified can be divided into three families on the basis of their different requirements for activation. Members of the conventional PKC family are Ca2+ and phospholipid-dependent and are activated by diacylglycerols and phorbol esters.
In vitro: Previous study found that bisindolylmaleimides carrying a straight-chain alkyl side-chain bearing a cationic substituent, such as Ro 31-7549 (bisindolylmaleimide VIII) and Ro 31-8220, showed a significant improvement in potency over the simple bisindolylmaleimides. For bisindolylmaleimide VIII, a further improvement in potency was obtained by restricting the position of the amine substituent. Moreove, unlike the indolocarbazole, staurosporine, which displayed 2-fold selectivity for PKC-β over the other examined isoenzymes, bisindolylmaleimide VIII exhibited a slight selectivity for PKC-α over the other isoenzymes. Compounds such as bisindolylmaleimide VIII carrying a straight-chain alkyl side-chain with the cationic substituent were found to be equipotent as inhibitors of PKC-β, PKC-γ and PKC-ε [1].
In vivo: Animal study found that, in neonatal rats, high glucose levels could induce the hypertrophy of cardiomyocytes. Ro-31-8220, a analog of bisindolylmaleimide VIII, was able to reverse the effect of high glucose on the cardiac myocytes,which might be through PKC/NF-κB/c-Fos pathway [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Wilkinson, S. E.,Parker, P.J. and Nixon, J.S. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochemistry Journal 294, 335-337 (1993).
[2] Zhang, W. B. et al. Reverse effect of protein kinase C inhibitor Ro-31-8220 on the hypertrophy of cardiomyocytes of neonatal rats induced by high glucose levels. Chinese Journal of Pathophysiology. 2009-08.
| Storage | Store at -20°C |
| M.Wt | 458.5 |
| Cas No. | 138516-31-1 |
| Formula | C24H22N4O2·C2H4O2 |
| Synonyms | BIM VIII,Ro 31-7549 |
| Solubility | Soluble in DMSO |
| Chemical Name | 3-[1-(3-aminopropyl)-1H-indol-3-yl]-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione, acetate |
| SDF | Download SDF |
| Canonical SMILES | CC(O)=O.C[n]1c(cccc2)c2c(C(C(N2)=O)=C(c3c[n](CCCN)c4c3cccc4)C2=O)c1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure