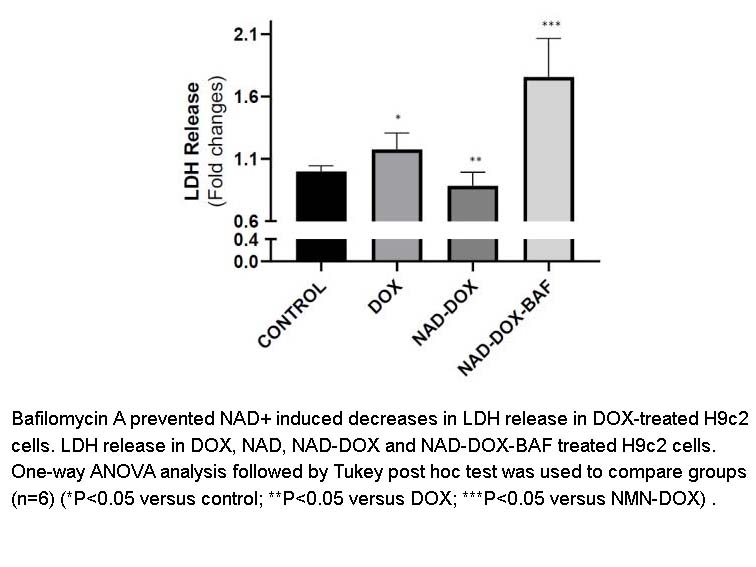
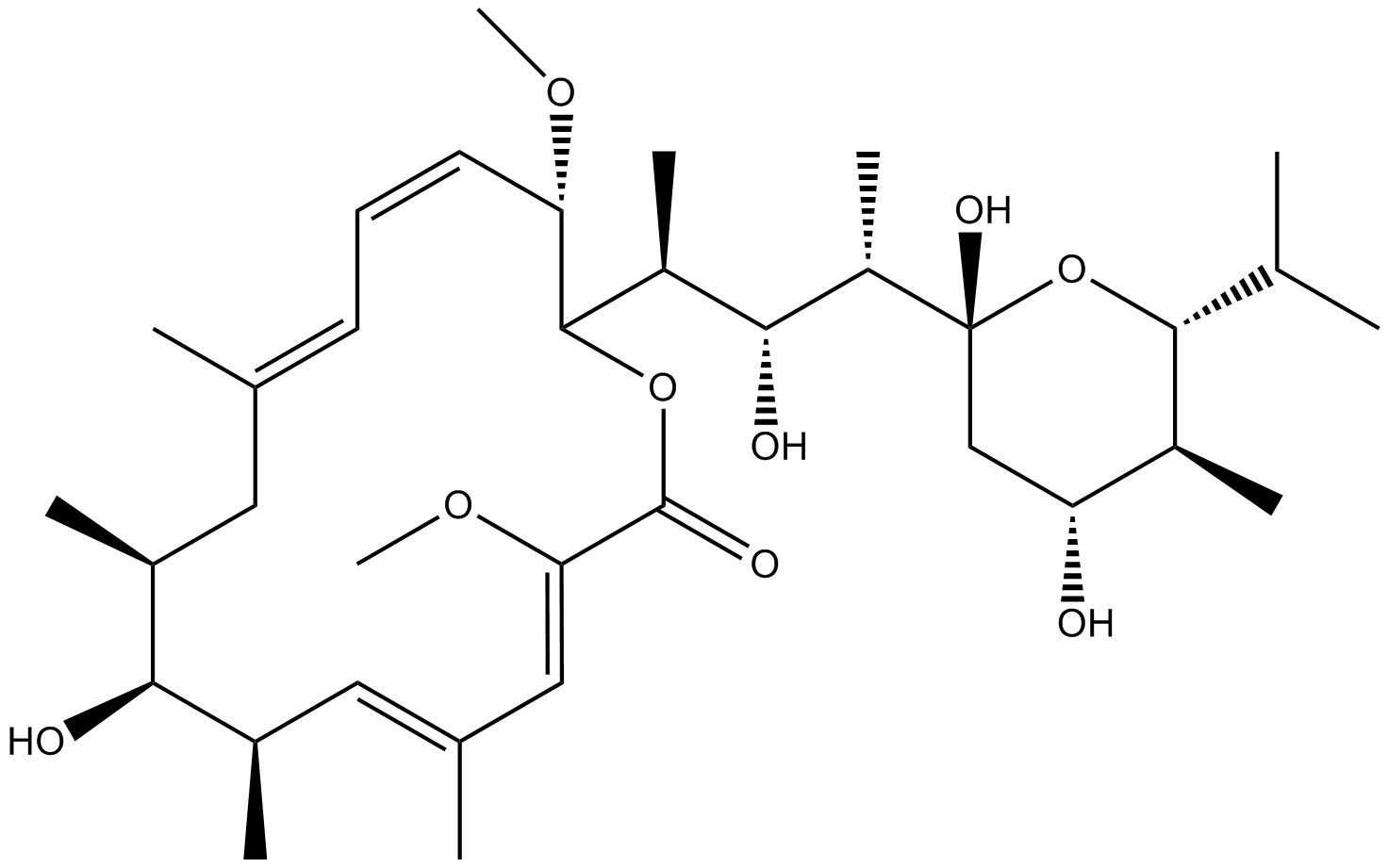
Bafilomycin A1
Bafilomycin A1(CAS 88899-55-2) is a selective inhibitor of vacuolar-type H⁺-ATPases (V-ATPases), enzymes responsible for proton translocation across organellar membranes. It inhibits V-ATPase enzymatic activity with established IC₅₀ values ranging from 4 to 400 nmol/mg protein, depending on the organismal source (plant, fungal, or animal origin). In vitro studies show Bafilomycin A1 completely blocks H⁺ transport through V-ATPases at concentrations as low as 10 nM. Additionally, this inhibitor is commonly employed in cell biology research to investigate roles of V-ATPases in intracellular pH regulation, lysosomal function, and osteoclast-mediated bone resorption.
References:
1. Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells[J]. Proceedings of the National Academy of Sciences, 1988, 85(21): 7972-7976.
2. Takami M, Suda K, Sahara T, et al. Involvement of vacuolar H+-ATPase in incorporation of risedronate into osteoclasts[J]. Bone, 2003, 32(4): 341-349.
- 1. Juan Jesus Vicente, Michael Wagenbach, Justin Decarreau. "The kinesin motor Kif9 regulates centriolar satellite positioning during interphase." Cell Press September 19, 2025 PMID: 40975050
- 2. Wenwen Yang, Meng Jia, et al. "Renal tubular VMP1 protects against acute kidney injury via modulating autophagy and autophagyindependent pathway." Autophagy. 2025 Jul 14 PMID: 40660473
- 3. Ruotong Yin, Yalin Wang, et al. "Promoting ubiquitin-dependent Drp1 degradation contributes to the protective effect of Astragalin against diabetic renal fibrosis." Biochem Pharmacol. 2025 Jul 12:241:117158 PMID: 40659133
- 4. Rebeka Butkovič, Michael D. Healy, et al. "Identification of a RAB32-LRMDA-Commander membrane trafficking complex reveals the molecular mechanism of human oculocutaneous albinism type 7." bioRxiv. 2025 Feb 4:2025.02.04.636395 PMID: 39975051
- 5. Ning Ding, Yijie Song, et al. "Heat-shock chaperone HSPB1 mitigates poly-glycine-induced neurodegeneration via restoration of autophagic flux." Autophagy. 2025 Feb 25:1-18 PMID: 39936620
- 6. Dongqi Nan, Chenglong Rao, et al. "Burkholderia pseudomallei BipD modulates host mitophagy to evade killing." Nat Commun. 2024 Jun 4;15(1):4740 PMID: 38834545
- 7. Juan Jesus Vicente, Michael Wagenbach, et al. "The kinesin motor Kif9 regulates centriolar satellite positioning and mitotic progression." bioRxiv. 2024 Apr 3:2024.04.03.587821 PMID: 38617353
- 8. Zijie Jin, Ruotong Yin, et al. "Dapagliflozin ameliorates hepatic steatosis via suppressing LXRα-mediated synthesis of lipids and bile acids." Biochem Pharmacol. 2024 May:223:116167 PMID: 38527558
- 9. Zeying Zhang, Di Yang, et al. "KPNB1-ATF4 induces BNIP3-dependent mitophagy to drive odontoblastic differentiation in dental pulp stem cells." Cell Mol Biol Lett 29, 145 (2024)
- 10. David Paul, Omer Stern, et al. "Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis." Nat Commun. 2023 Feb 28;14(1):947 PMID: 36854675
- 11. Qian Li, Dongqi Nan, et al. "Burkholderia pseudomallei BipD hijacks host KLHL9/KLHL13/CUL3 E3 ligase to ubiquitinate IMMT that initiates mitophagy to evade killing." Research Square. 15 Sep, 2023
- 12. Charlotte Nugues, Dayani Rajamanoharan, et al. "Lysosome exocytosis is required for mitosis in mammalian cells." Biochem Biophys Res Commun. 2022 Oct 20;626:211-219 PMID: 35998546
- 13. Yukang Wen, Zhengkun Chen, et al. "Incomplete autophagy promotes the proliferation of Mycoplasma hyopneumoniae through the JNK and Akt pathways in porcine alveolar macrophages." Vet Res. 2022 Aug 4;53(1):62 PMID: 35927699
- 14. Stavroula Zagkou, Valentine Marais, et al. "Design and Evaluation of Autophagy-Inducing Particles for the Treatment of Abnormal Lipid Accumulation." Pharmaceutics. 2022 Jun 29;14(7):1379 PMID: 35890275
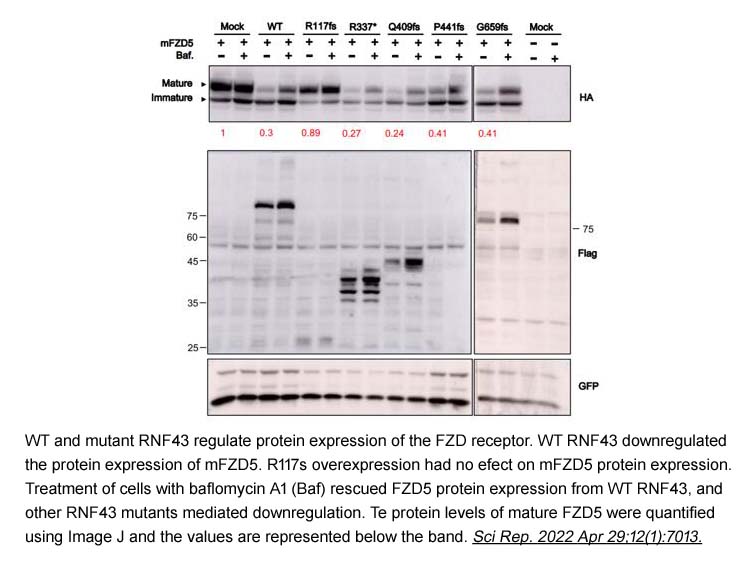
- 15. A‑Ri Cho, Hee Jung Sul, et al. "RNF43 R117fs mutant positively regulates Wnt/β-catenin signaling by failing to internalize FZD expressed on the cell surface." Sci Rep. 2022 Apr 29;12(1):7013 PMID: 35487932
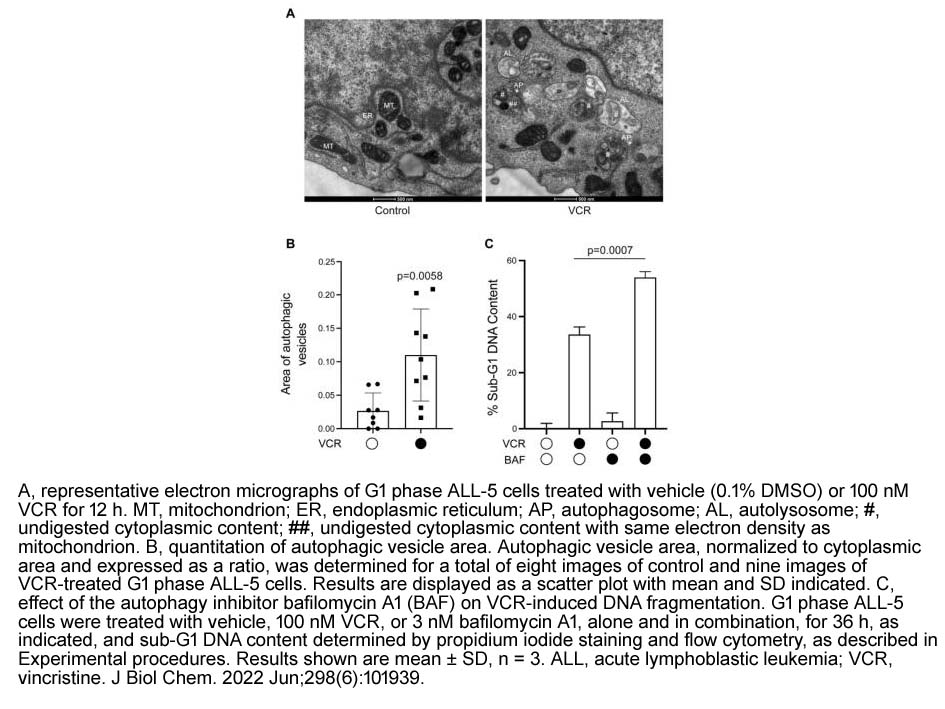
- 16. Magdalena Delgado, Randall R. Rainwater, et al. "Primary acute lymphoblastic leukemia cells are susceptible to microtubule depolymerization in G1 and M phases through distinct cell death pathways." J Biol Chem. 2022 Jun;298(6):101939 PMID: 35436470
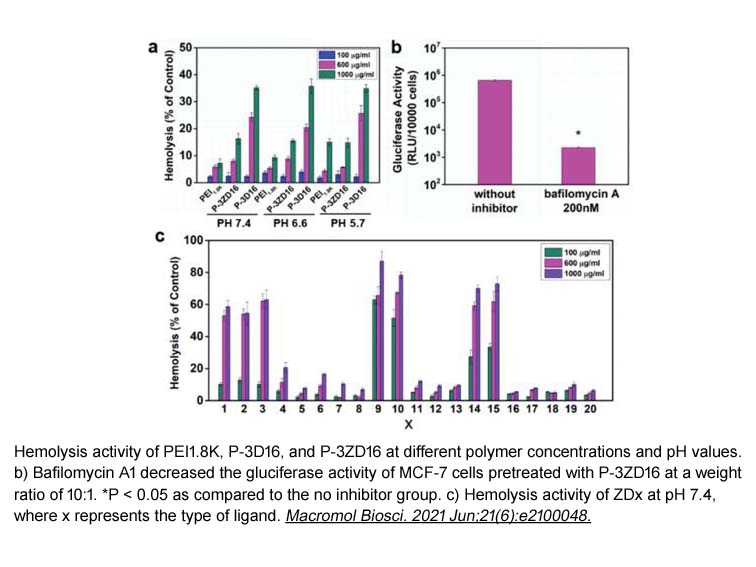
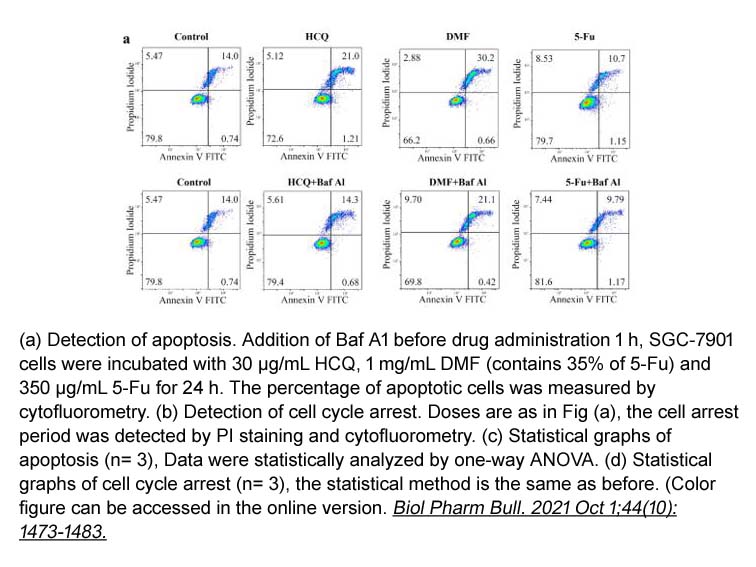
- 17. Chunhua Shu, Rui Wang, et al. "The." Dextran-Magnetic Layered Double Hydroxide-Fluorouracil" Drug Delivery System Exerts Its Anti-tumor Effect by Inducing Lysosomal Membrane Permeability in the Process of Cell Death." Biol Pharm Bull. 2021 Oct 1;44(10):1473-1483 PMID: 34305072
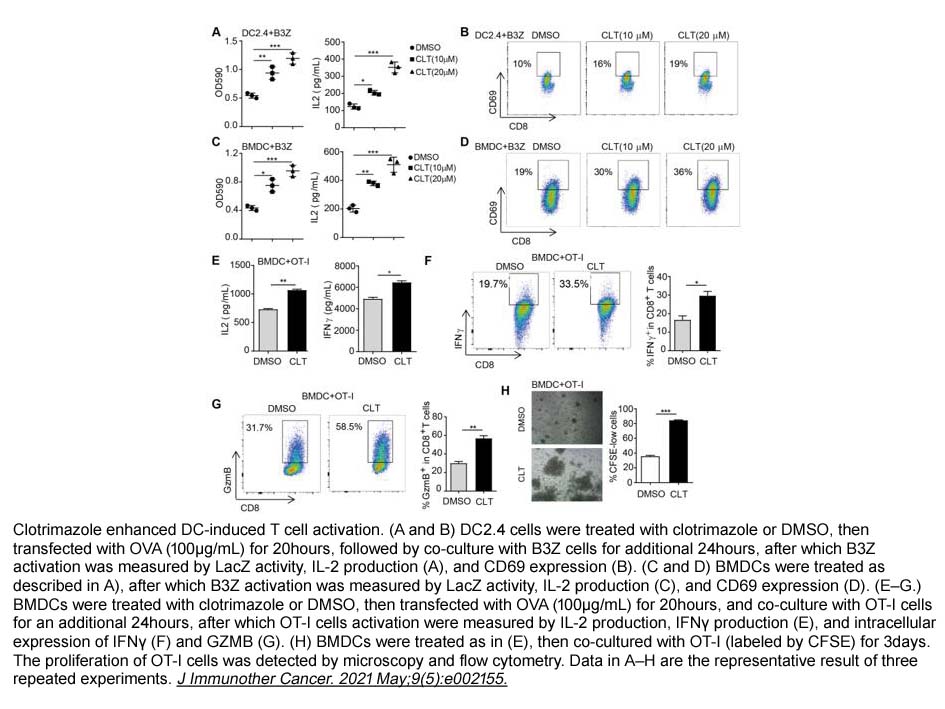
- 18. Zining Wang, Feifei Xu, et al. "Modulation of lactate-lysosome axis in dendritic cells by clotrimazole potentiates antitumor immunity." J Immunother Cancer. 2021 May;9(5):e002155 PMID: 34016722
- 19. Huiting Jia, Xindong Wang, et al. "Zinc(II)-Dipicolylamine Analogs Mediated PEI1.8k/pDNA Vector: Effect of Ligand Structure on the Gene Transport Process." Macromol Biosci. 2021 Jun;21(6):e2100048 PMID: 33861507
- 20. Zhuo Sun, Yidan Cao, et al. "Antiangiogenic effect of arsenic trioxide in HUVECs by FoxO3a‐regulated autophagy." J Biochem Mol Toxicol. 2021 Feb 16;e22728 PMID: 33592126
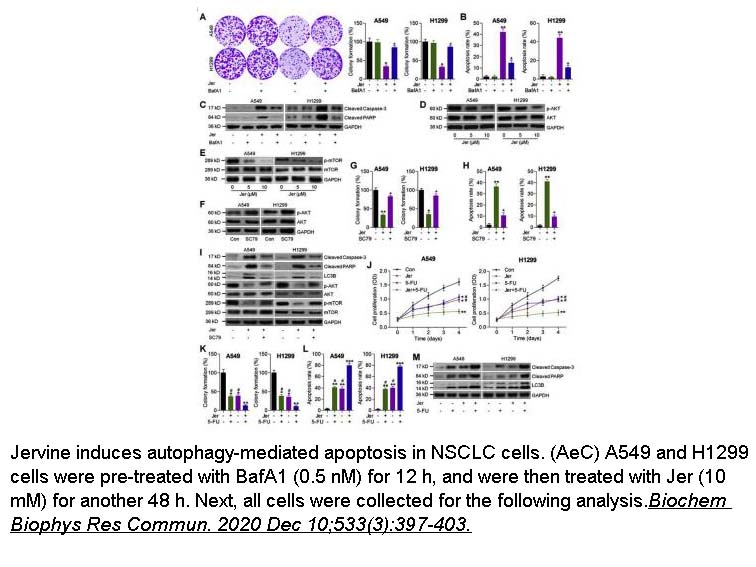
- 21. Wei Lei, Zhenyun Huo, et al. "Jervine inhibits non-small cell lung cancer (NSCLC) progression by suppressing Hedgehog and AKT signaling via triggering autophagy-regulated apoptosis." Biochem Biophys Res Commun. 2020 Dec 10;533(3):397-403 PMID: 32972750
- 22. Xie X, Yang C, et al. "Advanced glycation end products reduce macrophage-mediated killing of Staphylococcus aureus by ARL8 upregulation and inhibition of autolysosome formation." Eur J Immunol. 2020;10.1002/eji.201948477 PMID: 32250445
- 23. Wu L, Duan Q, et al. "Zearalenone Blocks Autophagy Flow and Induces Cell Apoptosis During Embryo Implantation in Gilts." Toxicol Sci. 2020;175(1):126-139 PMID: 32239165
- 24. Nima Nalin. "Nicotinamide mononucleotide imparts protection against Doxorubicin-induced cardiotoxicity by maintaining lysosomal acidification." The University of Western Ontario
- 25. Liang D, Wang HY, et al. "W941, a new PI3K inhibitor, exhibits preferable anti-proliferative activities against nonsmall cell lung cancer with autophagy inhibitors." Invest New Drugs. 2019 Dec 10 PMID: 31823159
- 26. Lin Tang, Xinxun Xiao, et al. "Induction of oocyte maturation and changes in the biochemical composition, physiology and molecular biology of oocytes during maturation and hydration in the orange-spotted grouper (Epinephelus coioides." aquaculture 4 December 2019. PII: S0044-8486(19)33325-3
- 27. Wang H, Liu W, et al. "Inhibitor analysis revealed that clathrin-mediated endocytosis is involed in cellular entry of type III grass carp reovirus." Virol J. 2018 May 24;15(1):92 PMID: 29793525
- 28. Xinchun Li, Li Zhong, et al. "Phosphorylation of IRS4 by CK1γ2 promotes its degradation by CHIP through the ubiquitin/lysosome pathway." Theranostics, 2018, Vol. 8, Issue13
- 29. Boyd Tressler, Andrea Michelle. "Mechanisms of Extracellular Nucleotide Accumulation During Regulated Cell Death in Tumor Cells." rave.ohiolink.edu,May 2016
- 30. Liu, Shuangxin, et al. "Bovine parathyroid hormone enhances osteoclast bone resorption by modulating V-ATPase through PTH1R." International journal of molecular medicine (2015) PMID: 26647715
- 31. Boyd-Tressler, Andrea, et al. "Chemotherapeutic Drugs Induce ATP Release via Caspase-gated Pannexin-1 Channels and a Caspase/Pannexin-1-Independent Mechanism." Journal of Biological Chemistry (2014): jbc-M114 PMID: 25112874
| Physical Appearance | A crystalline solid |
| Storage | Desiccate at -20°C |
| M.Wt | 622.84 |
| Cas No. | 88899-55-2 |
| Formula | C35H58O9 |
| Solubility | Soluble in DMSO |
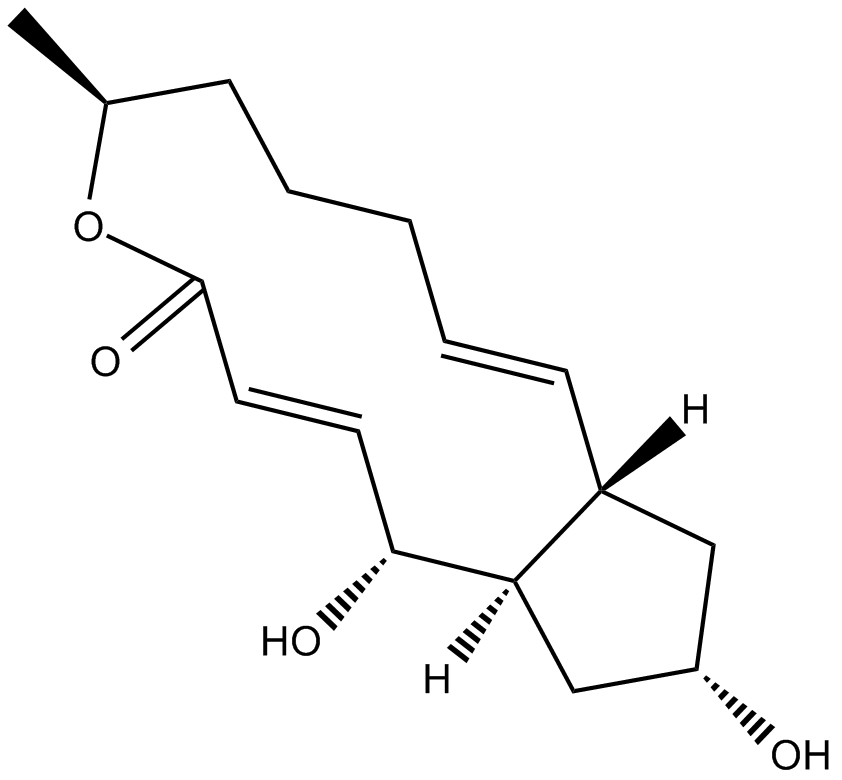
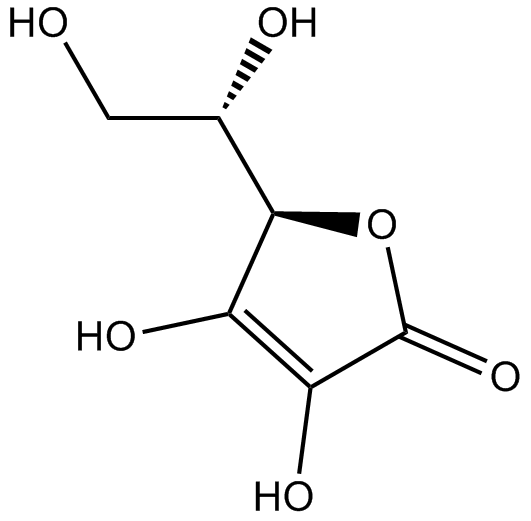
| Chemical Name | (3Z,5E,7R,8S,9S,11E,13E,15S,16R)-16-[(2S,3R,4S)-4-[(2R,4R,5S,6R)-2,4-dihydroxy-5-methyl-6-propan-2-yloxan-2-yl]-3-hydroxypentan-2-yl]-8-hydroxy-3,15-dimethoxy-5,7,9,11-tetramethyl-1-oxacyclohexadeca-3,5,11,13-tetraen-2-one |
| SDF | Download SDF |
| Canonical SMILES | CC1CC(=CC=CC(C(OC(=O)C(=CC(=CC(C1O)C)C)OC)C(C)C(C(C)C2(CC(C(C(O2)C(C)C)C)O)O)O)OC)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
HeLa cells |
|
Preparation method |
The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months. |
|
Reacting condition |
0 ~ 20 nM |
|
Applications |
Bafilomycin A1 dose-dependently inhibited the vacuolization of Hela cells induced by H. pylori, showing a 50% effect at 4 nM and a complete inhibition at 12.5 nM. In addition, Bafilomycin A1 also efficiently restored vacuolated cells to a normal appearance. |
| Animal experiment [2]: | |
|
Animal models |
Young freshwater tilapias |
|
Dosage form |
0 ~ 10-5 mol/L; 30 mins |
|
Applications |
In young tilapias, Bafilomycin A1 dose-dependently inhibited the rate of Na+ uptake with a Ki value of 1.6 × 10-7 mol/L. The inhibitory effect (20%) was observed at a concentration as low as 10-8 mol/L and increased linearly up to a concentration of 10-6 mol/L, after which it remained at approximately 90 % inhibition. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Papini E, Bugnoli M, De Bernard M, Figura N, Rappuoli R, Montecucco C. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993 Jan;7(2):323-7. [2]. Fenwick JC, Wendelaar Bonga SE, Flik G. In vivo bafilomycin-sensitive Na(+) uptake in young freshwater fish. J Exp Biol. 1999 Dec;202 Pt 24:3659-66. |
|
| Description | Bafilomycin A1 is a selective and reversible inhibitor of vacuolar H+ ATPases (V-ATPases) with IC50 value of 4-400 nM. | |||||
| Targets | V-ATPases | |||||
| IC50 | 4-400 nM | |||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data