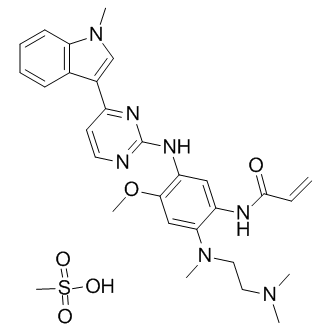
AZD-9291 mesylate
First-generation EGFR tyrosine kinase inhibitors (EGFR TKI) provide significant clinical benefit in patients with advanced EGFR-mutant (EGFRm) non-small cell lung cancer (NSCLC). Patients ultimately develop disease progression, usually due to the acquisition of the resistance mutation. AZD9291 is a oral, potent, and selective third generation irreversible inhibitor of both EGFRm sensitizing and T790M resistance mutants which spares wild-type EGFR.
In vitro: AZD9291 potently inhibits signaling pathways and cellular growth in both EGFRm and EGFRm/T790M mutant cell lines, with lower activity against WT EGFR cell lines. AZD9291 showed an apparent IC50 of 12 nmol/L against L858R and 1 nmol/L against L858R/T790M EGFRm [1].
In vivo: AZD9291 demonstrates good bioavailability, is widely distributed in tissues, and has moderate clearance resulting in a half-life of around 3 hours after oral dosing in the mouse . Once-daily dosing of AZD9291 induced significant dose-dependent regression in both PC-9 (ex19del) and H1975 (L858R/T790M) tumor xenograft models. The tumor shrinkage was observed at doses low to 2.5 mg kg-1 day-1 in both models [1].
Clinical trial: The mesylate salt of AZD9291 is currently in a first-in-human phase I dose-escalation clinical trial (AURA; NCT01802632; AstraZeneca) in patients with advanced EGFRm NSCLC who had disease progression following treatment with any EGFR TKI (including gefitinib or erlotinib). AZD9291 is showing promising responses in this phase I trial even at the first-dose level.
Reference:
[1] Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046-61.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 595.71 |
| Cas No. | 1421373-66-1 |
| Formula | C29H37N7O5S |
| Solubility | insoluble in EtOH; insoluble in DMSO; insoluble in H2O |
| Chemical Name | (Z)-N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylimidic acid compound with methanesulfonic acid (1:1) |
| SDF | Download SDF |
| Canonical SMILES | C=C/C(O)=N/C1=CC(NC2=NC=CC(C(C3=CC=CC=C34)=CN4C)=N2)=C(OC)C=C1N(CCN(C)C)C.CS(O)(=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure