AR-C155858
AR-C155858 is a potent and selective inhibitor of monocarboxylate transporters MCT1 and MCT2 with Ki values of 2.3nM and less than 10 nM, respectively [1].
MCT is a monocarboxylate transporter family with 14 members. Among these transporters, MCT1 and MCT2 are worked for the uptake and efflux of lactic acid. As a selective inhibitor of MCT1 and MCT2, AR-C155858 can be used to probe MCTs’ roles in the metabolic studies. Besides that, AR-C155858 was found to have immunosuppressive activity and can inhibit the proliferation of T-lymphocytes. The binding site of MCT1 for AR-C155858 contains transmembrane helices 7-10 in C-terminal domain. The two residues Phe360 and Ser364 play important roles in the binding [1 and 2].
In rat erythrocytes in which the AE1 mediated lactate transport had been blocked, AR-C155858 inhibited endogenous MCT1 mediated L-lactate uptake dose-dependently. AR-C155858 showed to be a tight-binding non-competitive inhibitor with Ki value of 2.3±1.4 nM and Kcat value of 12.2±1.1 s-1.In Xenopus oocytes expressing MCT1, MCT2 or MCT4, AR-C155858 at concentration of 100 nM showed 100% inhibition of MCT1 mediated lactate uptake. For MCT2, AR-C155858 at 10 nM showed 70% inhibition. AR-C155858 had no significant effect against MCT4 even at concentration up to 10 μM. In addition, it has been found that the inhibition of MCTs caused by AR-C155858 can be affected by the co-expression of ancillary proteins. The presence of embigin reduced the sensitivity of MCT2 against AR-C155858’s inhibition. AR-C155858 potently suppressed the uptake of lactic acid in Ras-transformed fibroblast CCL39 cells via inhibiting MCT1 and MCT2 but no MCT4. The suppression subsequently resulted in significant decrease of glycolysis [1, 3 and 4].
In nude mice implanted with Ras-transformed CCL39 fibroblasts, the administration of AR-C155858 at dose of 30 mg/kg twice daily resulted in significant tumor growth suppression [4].
References:
[1] Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10. Biochem J. 2010 Jan 15;425(3):523-30.
[2] Nancolas B, Sessions RB, Halestrap AP. Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity. Biochem J. 2015 Feb 15;466(1):177-88.
[3] Ovens MJ, Manoharan C, Wilson MC, Murray CM, Halestrap AP. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochem J. 2010 Oct 15;431(2):217-25.
[4] Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16663-8.
| Physical Appearance | White solid |
| Storage | Store at -20°C |
| M.Wt | 461.53 |
| Cas No. | 496791-37-8 |
| Formula | C21H27N5O5S |
| Synonyms | AR C155858 |
| Solubility | insoluble in H2O; ≥52.5 mg/mL in DMSO; ≥57.8 mg/mL in EtOH |
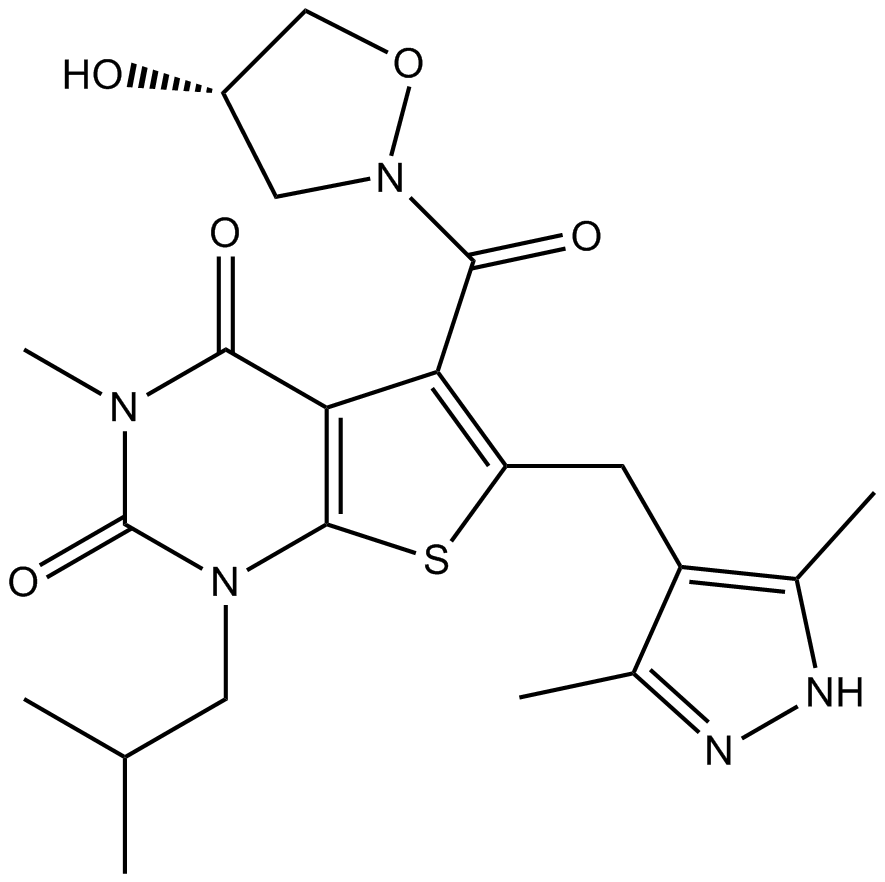
| Chemical Name | 6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-[(4S)-4-hydroxy-1,2-oxazolidine-2-carbonyl]-3-methyl-1-(2-methylpropyl)thieno[2,3-d]pyrimidine-2,4-dione |
| SDF | Download SDF |
| Canonical SMILES | CC(C)CN(c([s]c(Cc1c(C)[nH]nc1C)c1C(N(C2)OC[C@H]2O)=O)c1C(N1C)=O)C1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Ras-transformed fibroblast CCL39 cells |
|
Preparation method |
The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months. |
|
Reacting condition |
100 nM |
|
Applications |
In Ras-transformed fibroblast CCL39 cells, AR-C155858 potently suppressed the uptake of lactic acid by inhibiting MCT1 and MCT2, which significantly decreased glycolysis. |
| Animal experiment [2]: | |
|
Animal models |
Nude mice implanted with Ras-transformed CCL39 fibroblasts |
|
Dosage form |
30 mg/kg; s.c.; b.i.d., for 6 days |
|
Applications |
In nude mice implanted with Ras-transformed CCL39 fibroblasts that only expressed MCT1/2, AR-C155858 increased intracellular lactate pool, reduced glycolysis and growth in hypoxia, as well as inhibited tumor growth. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16663-8. |
|
Quality Control & MSDS
- View current batch:
Chemical structure