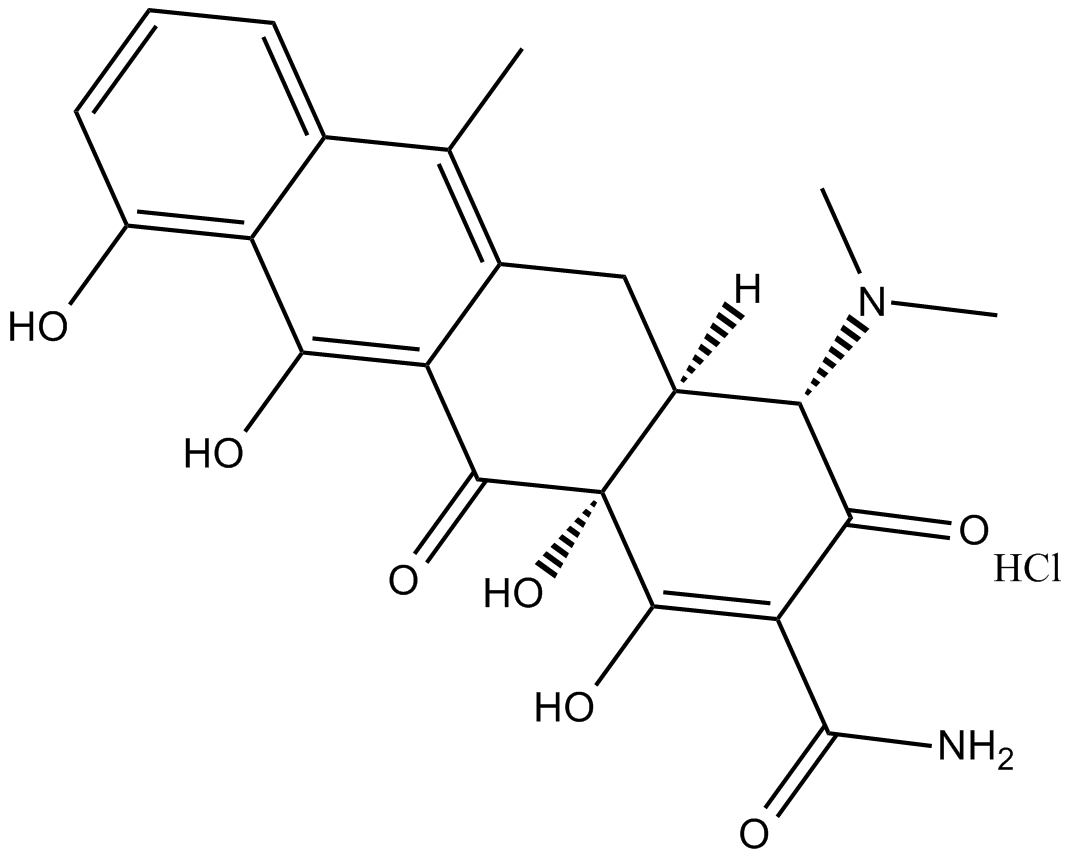
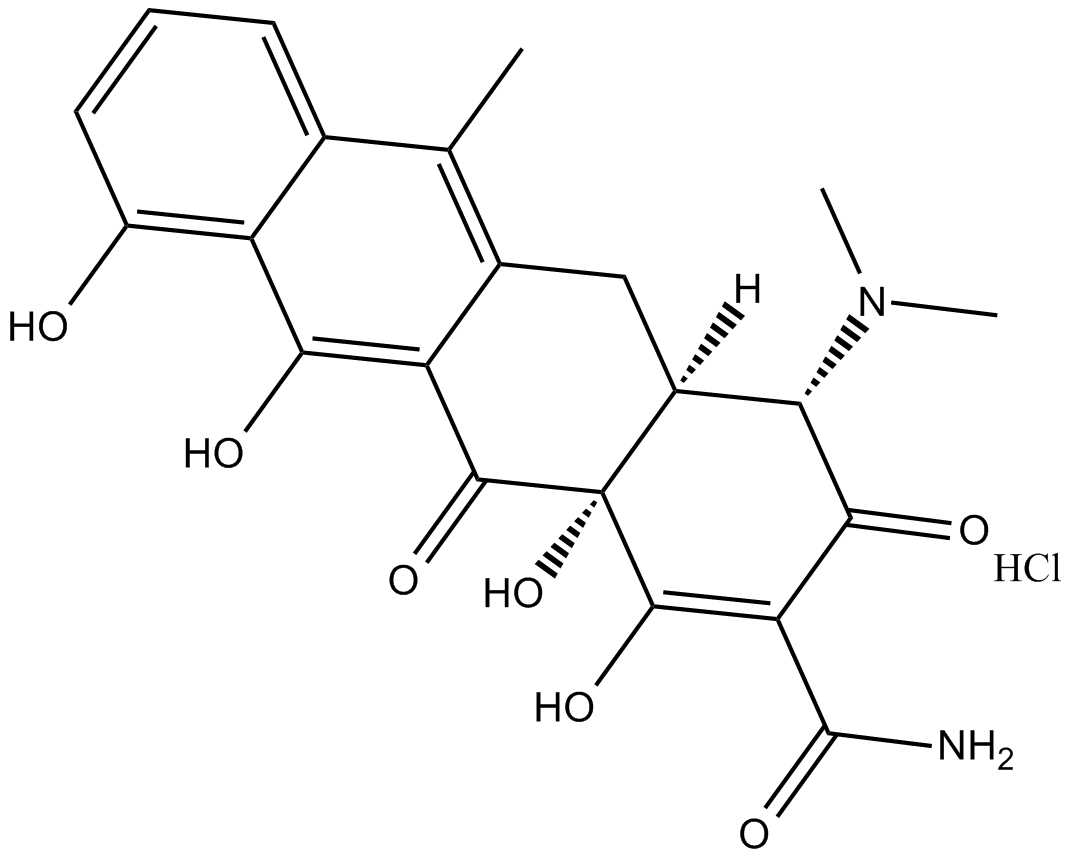
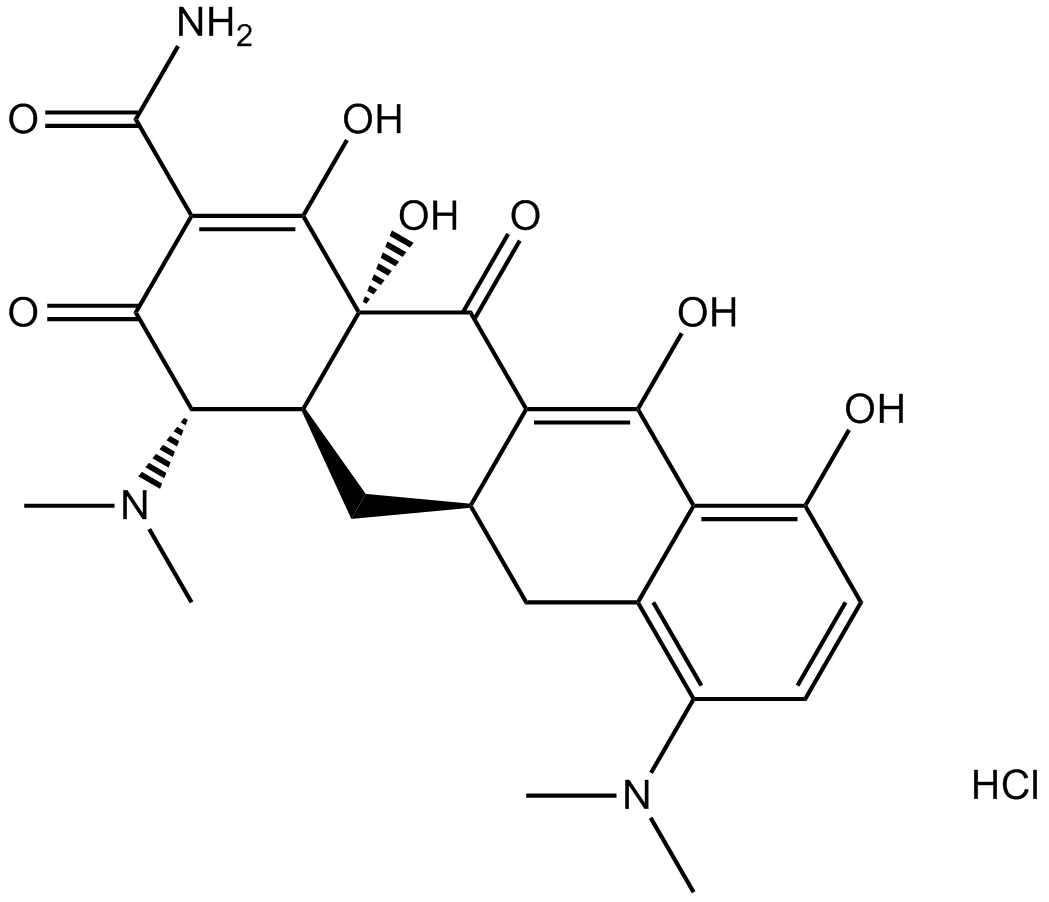
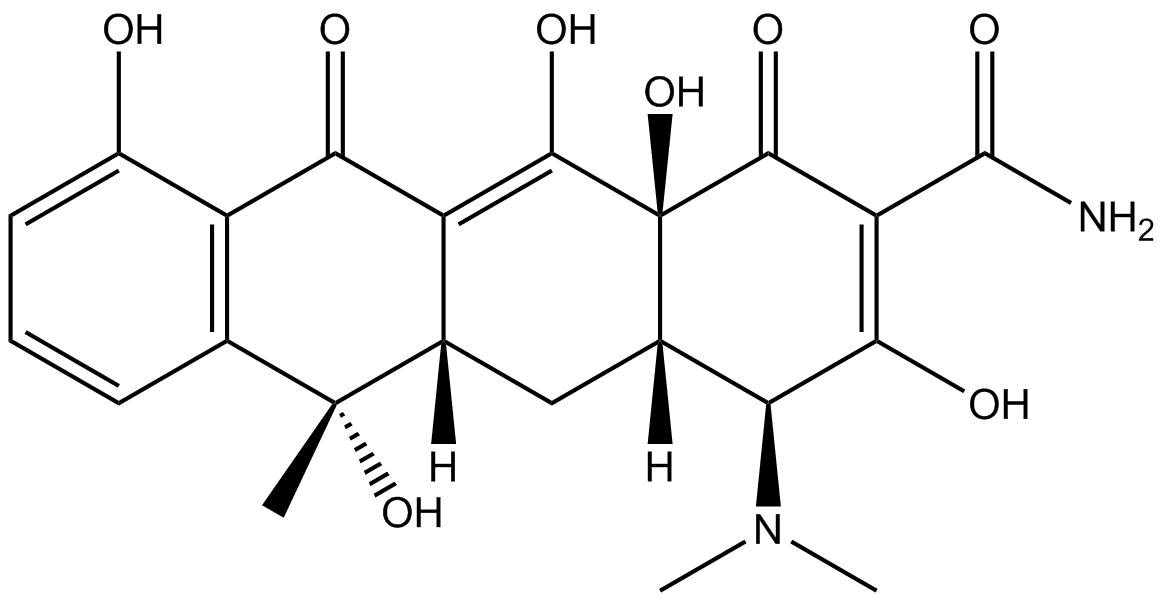
Anhydrotetracycline (hydrochloride)
Anhydrotetracycline hydrochloride (ATC;CAS 13803-65-1) is an effector molecule utilized in tetracycline-controlled gene expression systems, modulating the DNA-binding activity of tetracycline repressor protein (TetR) and its engineered variant reverse TetR (revTetR). TetR binds to operator DNA sequences, inhibiting transcription. ATC binding induces TetR conformational changes, dissociating it from operator DNA, enabling gene expression. Conversely, ATC acts as a corepressor in revTetR systems, enhancing its DNA-binding inhibition. ATC displays approximately 35-fold greater affinity towards TetR compared to tetracycline, with an IC50 near 0.4 μM, employed in inducible gene expression studies in biological research contexts.
References:
[1]. Gossen M, Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Res. 1993 Sep 11;21(18):4411-2.
[2]. Kamionka A, Bogdanska-Urbaniak J, Scholz O, et al. Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor. Nucleic Acids Res. 2004 Feb 4;32(2):842-7.
[3]. Resch M, Striegl H, Henssler EM, et al. A protein functional leap: how a single mutation reverses the function of the transcription regulator TetR. Nucleic Acids Res. 2008 Aug;36(13):4390-401.
[4]. Degenkolb J, Takahashi M, Ellestad GA, et al. Structural requirements of tetracycline-Tet repressor interaction: determination of equilibrium binding constants for tetracycline analogs with the Tet repressor. Antimicrob Agents Chemother. 1991 Aug;35(8):1591-5.
- 1. Dr. Ola Abdalla, Professor. Cameron Walker. "Advanced Fluctuation Assay and R-Based Analysis Protocol for Estimating Mutation Rates of H3K9-Methylated Heterochromatin." Biological and Medicinal Chemistry 11 September 2025
- 2. Matthew R Kent, Amanda N Jay, et al. "New dual inducible cellular model to investigate temporal control of oncogenic cooperating genes." Sci Rep. 2024 Sep 5;14(1):20773 PMID: 39237585
- 3. Runtao Zhu1, Zhuojun Dai, et al. "Engineering functional materials through bacteria-assisted living grafting." Cell Syst. 2024 Mar 20;15(3):264-274.e9 PMID: 38460522
- 4. Yunfu Lu, Feifei Chen, et al. "Modulation of MRSA virulence gene expression by the wall teichoic acid enzyme TarO." Nat Commun. 2023 Mar 22;14(1):1594 PMID: 36949052
| Storage | Store at -20°C |
| M.Wt | 462.9 |
| Cas No. | 13803-65-1 |
| Formula | C22H22N2O7·HCl |
| Solubility | ≥12.5 mg/mL in H2O; ≥15.43 mg/mL in EtOH with gentle warming; ≥2.42 mg/mL in DMSO with ultrasonic |
| Chemical Name | 4-(dimethylamino)-1,4S,4aS,5,12,12aS-hexahydro-3,10,11,12a-tetrahydroxy-6-methyl-1,12-dioxo-monohydrochloride-2-naphthacenecarboxamide |
| SDF | Download SDF |
| Canonical SMILES | Cc(c1c2c(O)ccc1)c(C[C@@H]([C@@H](C(OCl)=C(C(N)=O)C1=O)N(C)C)[C@@]1(C1=O)O)c1c2O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
HeLa XI cells |
|
Reaction Conditions |
0 ~ 100 ng/ml anhydrotetracycline hydrochloride for 4 d incubation |
|
Applications |
Anhydrotetracycline was much more effective than tetracycline in inactivating transcriptional transactivator (tTA), and completely abolished tTA mediated luciferase activity at concentrations as low as 3 ng/ml. More importantly, the concentration at which the prolonged presence of anhydrotetracycline began to affect the growth rate of HeLa cells in culture (> 3 μg/ml) was more than thousand fold above the effective concentration. In addition, like tetracycline, anhydrotetracycline was well suited to attenuate gene expression at intermediate levels of induction. These properties together with the high functional stability of anhydrotetracycline in cell culture made anhydrotetracycline a most attractive alternative effector for tetracycline-controlled gene expression in higher eukaryotic systems. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Gossen M, Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Research, 1993, 21(18): 4411-4412. |
|
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure