6H05
6H05 is a selective inhibitorof the common oncogenic mutant K-Ras (G12C). 6H05 allosterically modifies and inhibits the oncogenic G12C mutant of highly homologous protein H-Ras, not affecting the wild-type K-Ras [1].
K-Ras plays an important role in human cancer, cause mutations in K-Ras are the most common activating lesions in human cancer[2]. 6H05 modifies the GDP-bound K-Ras (G12C) the most, which can be and be applied as the starting point for drug-discovery efforts targeting K-Ras (G12C) and eventually other alleles of K-Ras. 6H05 can be used as an intermediate for the synthesis of oncogenic K-Ras (G12C) inhibitors [3, 4].
Although it’s necessary to perform continued chemical optimization of 6H05 to be assessed in vivo, preliminary evaluation of 6H05 in lung cancer cell lines suggests that 6H05 shows allele-specific impairment of K-Ras function[1]. There are questions still need to be illustrated that the selectivity and efficiency of 6H05 in vivo, as well as its effects on the subcellular localization of other farnesylated GTPases should be assessed further[1, 3].
Questions still need to be answered surrounding the in vivo selectivity and efficacy of 6H05, including its effects on the subcellular localization of other farnesylated GTPases [3].
References:
[1] Ostrem JM, Peters U, Sos ML, et al. K-Ras (G12C) inhibitors allosterically control GTP affinity and effector interactions[J]. Nature, 2013, 503(7477): 548-551.
[2] McCormick F. KRAS as a Therapeutic Target[J]. Clinical Cancer Research, 2015, 21(8): 1797-1801.
[3] Milroy L-G,Ottmann C. The Renaissance of Ras[J]. ACS chemical biology, 2014, 9(11): 2447-2458.
[4] Lu SY, Li S,Zhang J. Harnessing Allostery: A Novel Approach to Drug Discovery[J]. Medicinal Research Reviews, 2014, 34(6): 1242-1285.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 590.14 |
| Cas No. | 1469338-01-9 |
| Formula | C22H31ClF3N3O4S3 |
| Solubility | ≥29.5 mg/mL in DMSO; ≥14.3 mg/mL in H2O; ≥24.15 mg/mL in EtOH |
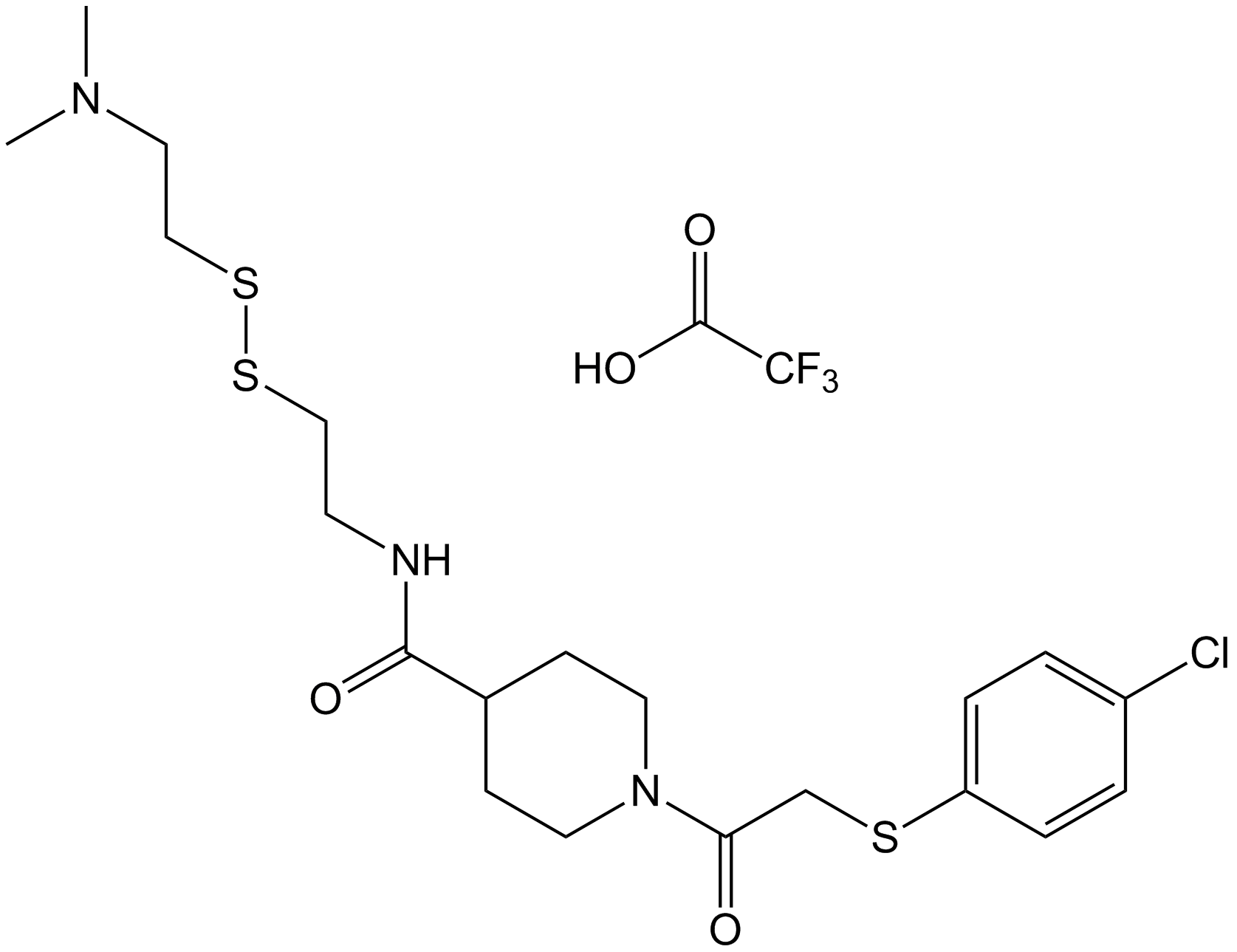
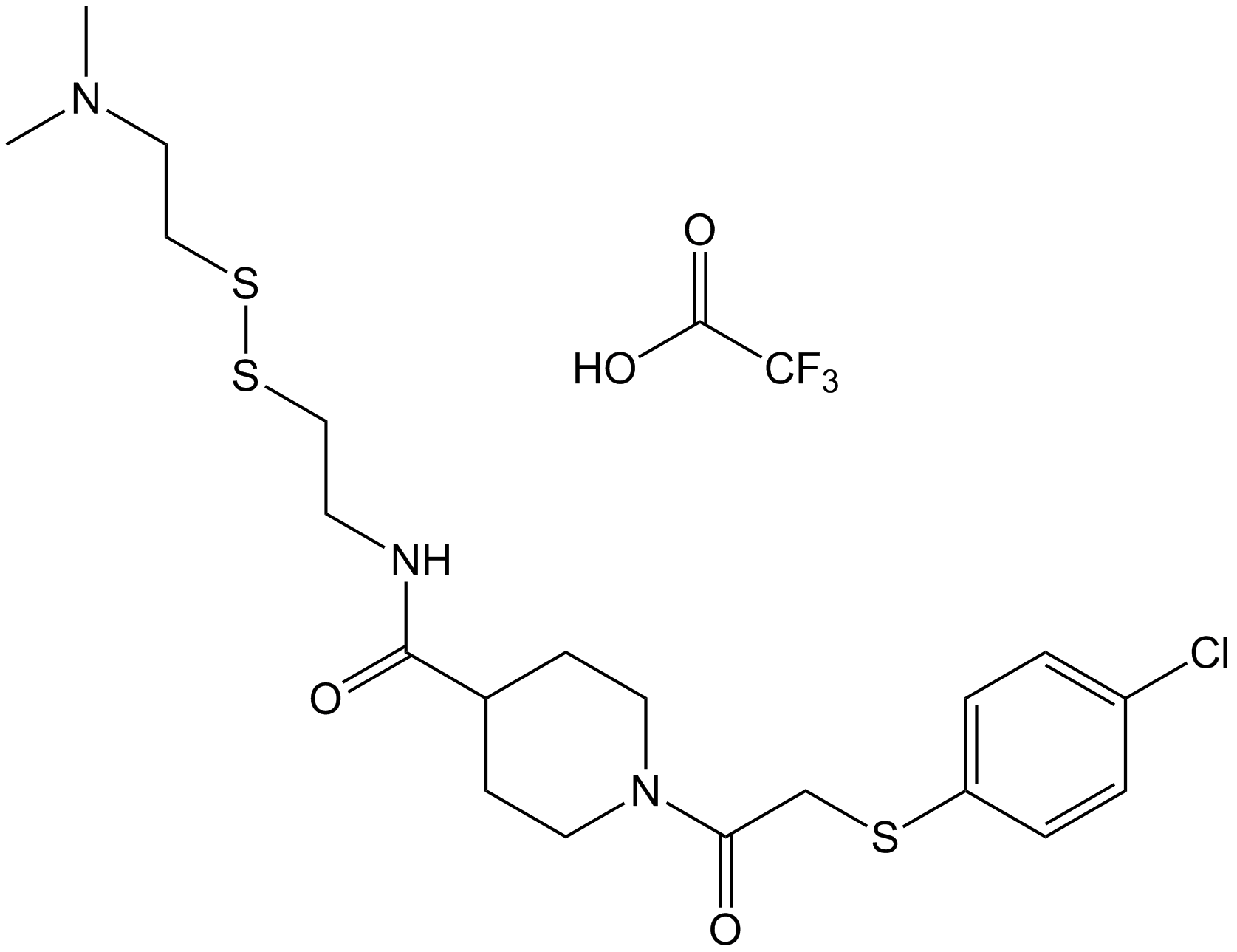
| Chemical Name | 1-(2-((4-chlorophenyl)thio)acetyl)-N-(2-((2-(dimethylamino)ethyl)disulfanyl)ethyl)piperidine-4-carboxamide 2,2,2-trifluoroacetate |
| SDF | Download SDF |
| Canonical SMILES | ClC1=CC=C(SCC(N2CCC(C(NCCSSCCN(C)C)=O)CC2)=O)C=C1.OC(C(F)(F)F)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure