2-D08
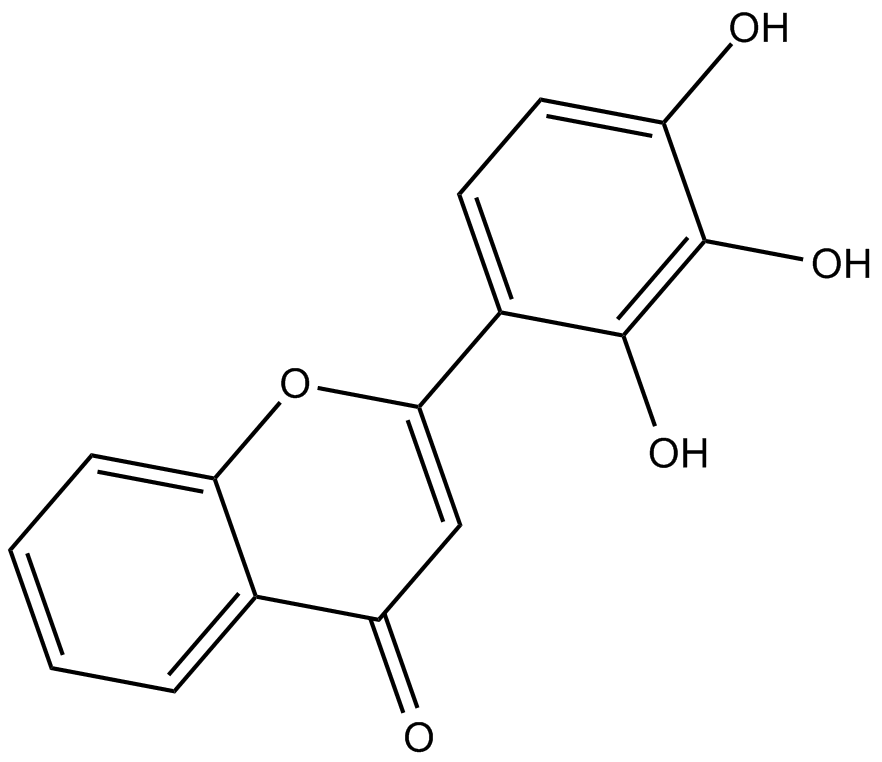
2-D08 (2’,3’,4’-trihydroxyflavone) is a Sumoylation inhibitor.
Protein sumoylation is a dynamic posttranslational modification involved in various biological processes, such as cellular homeostasis and development. Sumoylation has been reported to play a key role in cancer, though so far there are few small molecule probes available.
In vitro: 2-D08 was identified as a cell permeable, mechanistically unique inhibitor of protein sumoylation. This compound was found to be able to block sumoylation of topoisomerase I in two different cancer cell lines when co-dosed with camptothecin. In addition, futher analyses indicated that 2-D08 inhibited sumoylation via preventing transfer of small ubiquitin-like modifier (SUMO) from the UBC9-SUMO thioester to the substrate without affecting SUMO-activating enzyme E1 (SAE-1/2) or E2 Ubc9-SUMO thioester formation, a mechanism of action that was unprecedented before [1]. Moreover, it was found that 2-D08 at 100 μM could effectively inhibit 10 μM Camptothecin induced Topoisomerase I SUMOylation in breast cancer without affecting overall cellular protein ubiquitinations [2].
In vivo: Up to now, there is no animal in vivo data reported for 2-D08.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Kim YS, Keyser SG, Schneekloth JS Jr. Synthesis of 2',3',4'-trihydroxyflavone (2-D08), an inhibitor of protein sumoylation. Bioorg Med Chem Lett. 2014 Feb 15;24(4):1094-7.
[2] Kim YS, Nagy K, Keyser S, Schneekloth JS Jr. An electrophoretic mobility shift assay identifies a mechanistically unique inhibitor of protein sumoylation. Chem Biol. 2013 Apr 18;20(4):604-13.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 270.2 |
| Cas No. | 144707-18-6 |
| Formula | C15H10O5 |
| Solubility | ≥74.6 mg/mL in DMSO; ≥1.76 mg/mL in EtOH with gentle warming and ultrasonic; insoluble in H2O |
| Chemical Name | 2-(2,3,4-trihydroxyphenyl)-4H-1-benzopyran-4-one |
| SDF | Download SDF |
| Canonical SMILES | Oc(ccc(C(Oc1c2cccc1)=CC2=O)c1O)c1O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure