WEHI-539 hydrochloride
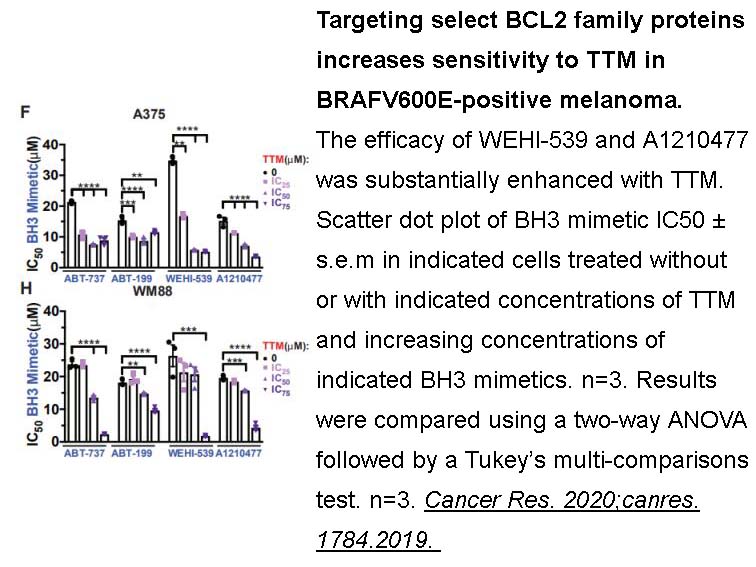
- 1. Kim YJ, Tsang T, et al. "Inhibition of BCL2 family members increases the efficacy of copper chelation in BRAFV600E-driven melanoma." Cancer Res. 2020;canres.1784.2019. PMID:32005716
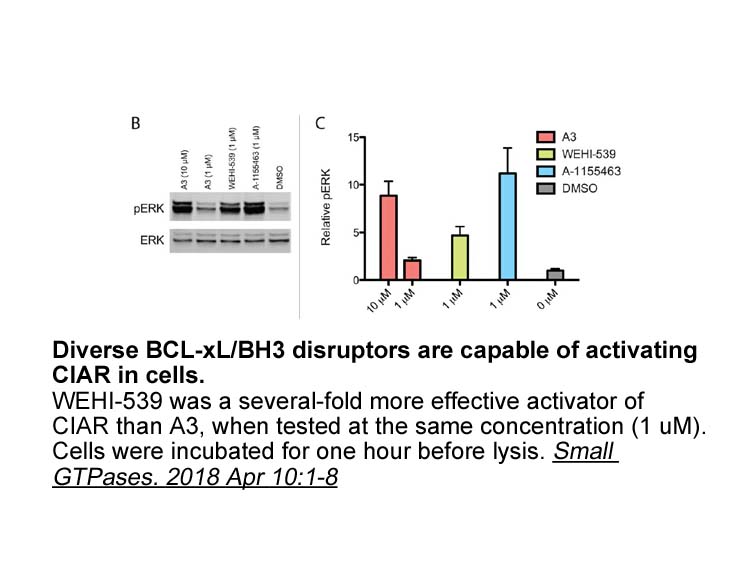
- 2. Rose JC, Dieter EM, et al. "Examining RAS pathway rewiring with a chemically inducible activator of RAS." Small GTPases. 2018 Apr 10:1-8. PMID:29634387
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 620.18 |
| Cas No. | 2070018-33-4 |
| Formula | C31H30ClN5O3S2 |
| Solubility | ≥28.55 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
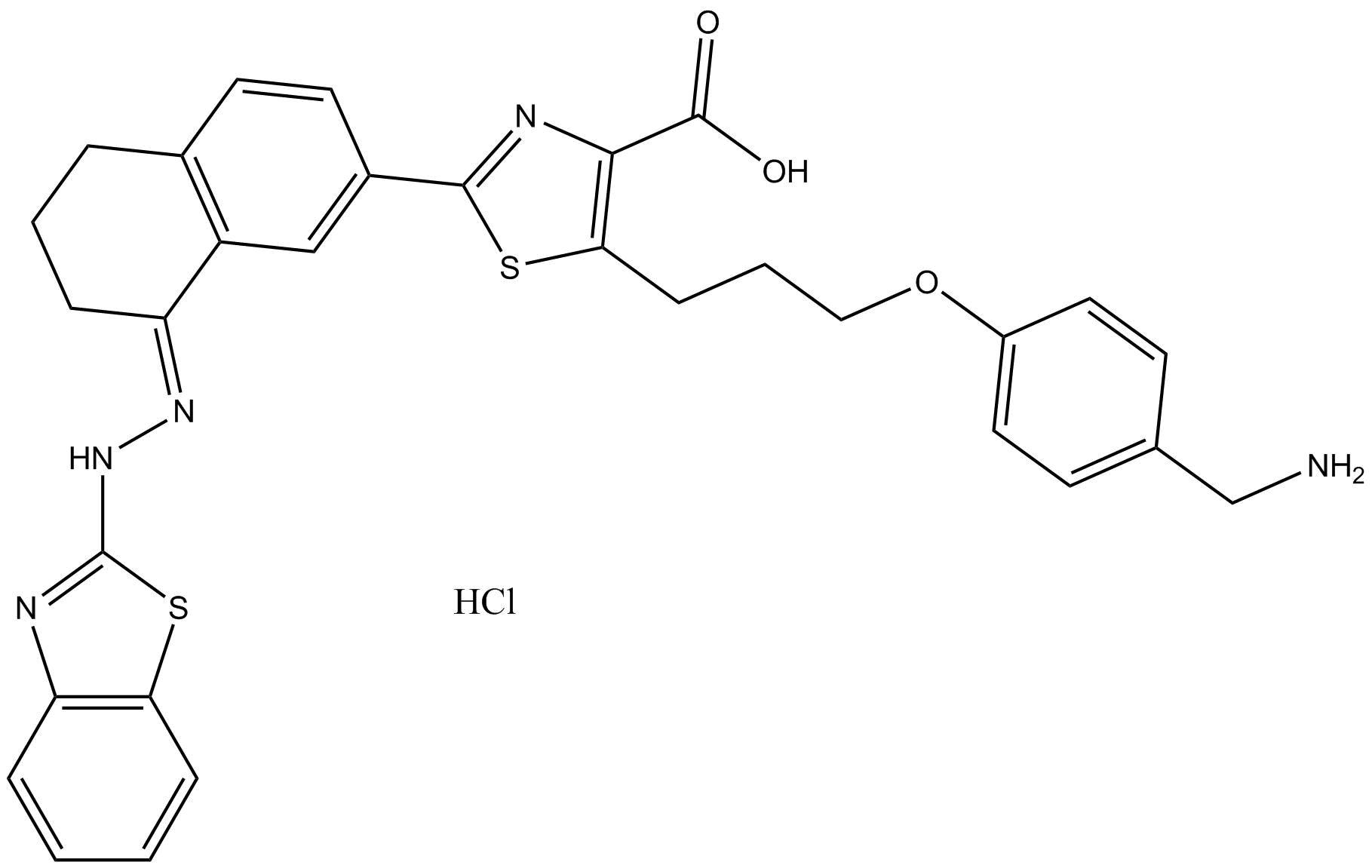
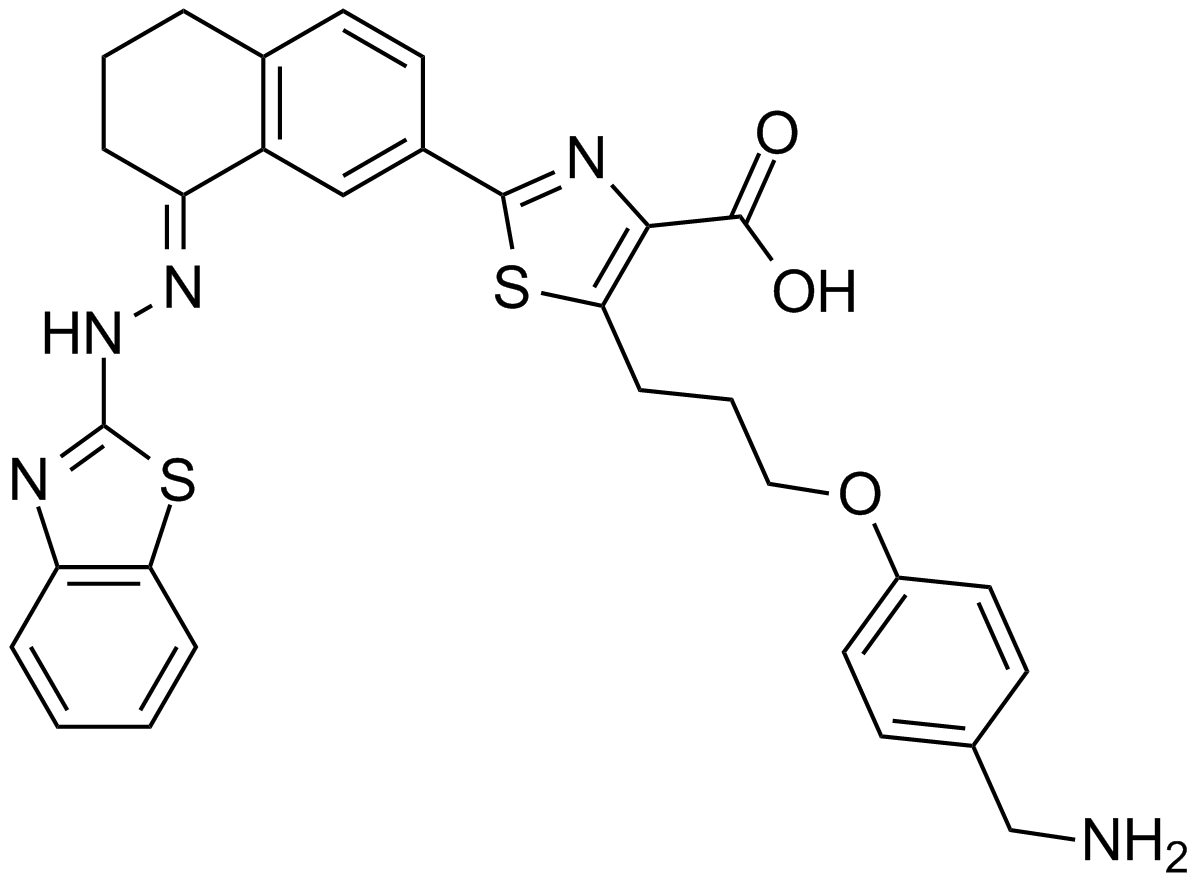
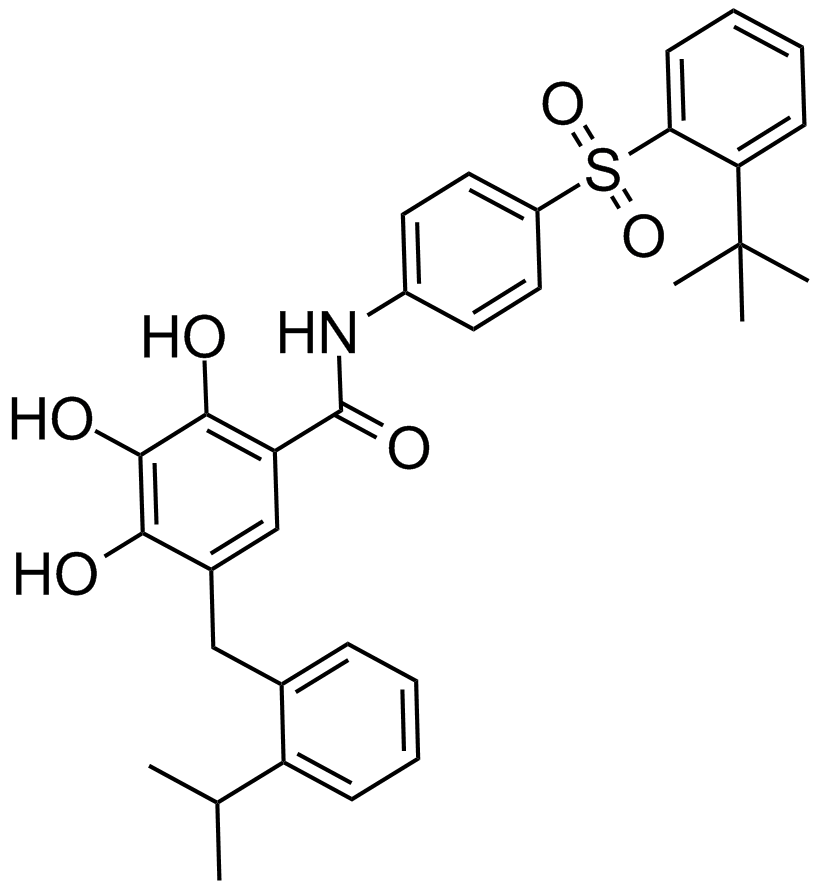
| Chemical Name | (E)-5-(3-(4-(aminomethyl)phenoxy)propyl)-2-(8-(2-(benzo[d]thiazol-2-yl)hydrazono)-5,6,7,8-tetrahydronaphthalen-2-yl)thiazole-4-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | O=C(C1=C(C([H])([H])C([H])([H])C([H])([H])OC2=C([H])C([H])=C(C([H])([H])N([H])[H])C([H])=C2[H])SC(C3=C([H])C4=C(C([H])=C3[H])C([H])([H])C([H])([H])C([H])([H])/C4=N\N([H])C5=NC6=C([H])C([H])=C([H])C([H])=C6S5)=N1)O[H] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment[1]: | |
|
Cell lines |
Human colon cancer cell |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
1 μM, 24h |
|
Applications |
Limiting dilution analysis with CSCs that were pre-treated with ABT-737, ABT-199 or WEHI-539 revealed that ABT-737 and WEHI-539 both were sufficient to decrease clonogenic capacity, whereas ABT-199 did not affect clonogenic growth. As WEHI-539 is selective for BCLXL, this points to a dependency of CSCs on BCLXL for survival. Importantly, ABT-737- or WEHI-539-induced loss of clonogenicity could be restored when BCLXL was ectopically overexpressed. When spheroid cultures were treated with ABT-737 or WEHI-539 compounds, CSCs were effectively sensitized toward oxaliplatin and other chemotherapeutic agents. |
|
References: 1. Colak S, Zimberlin CD, Fessler E et al. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death Differ. 2014 Jul;21(7):1170-7. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data