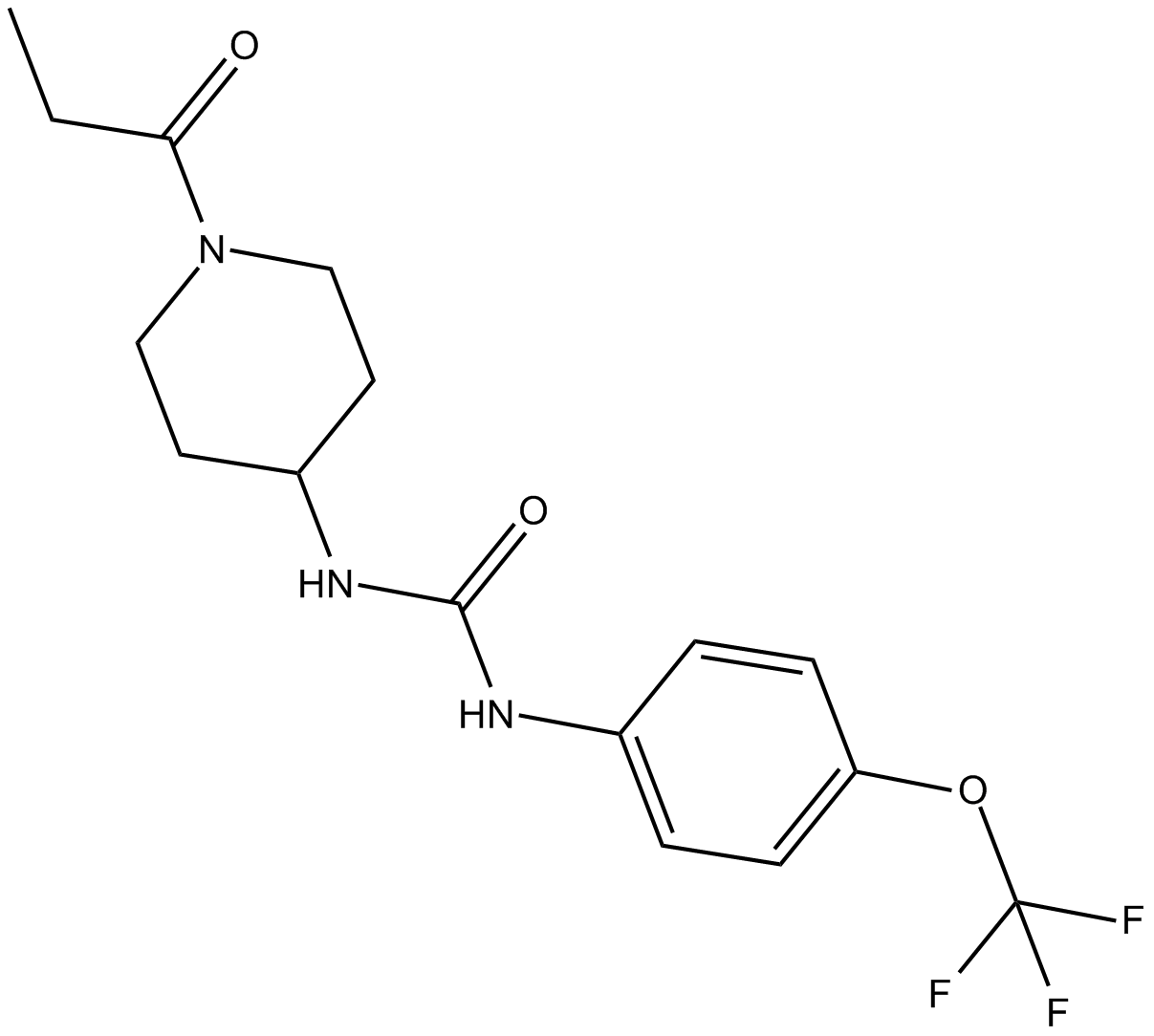
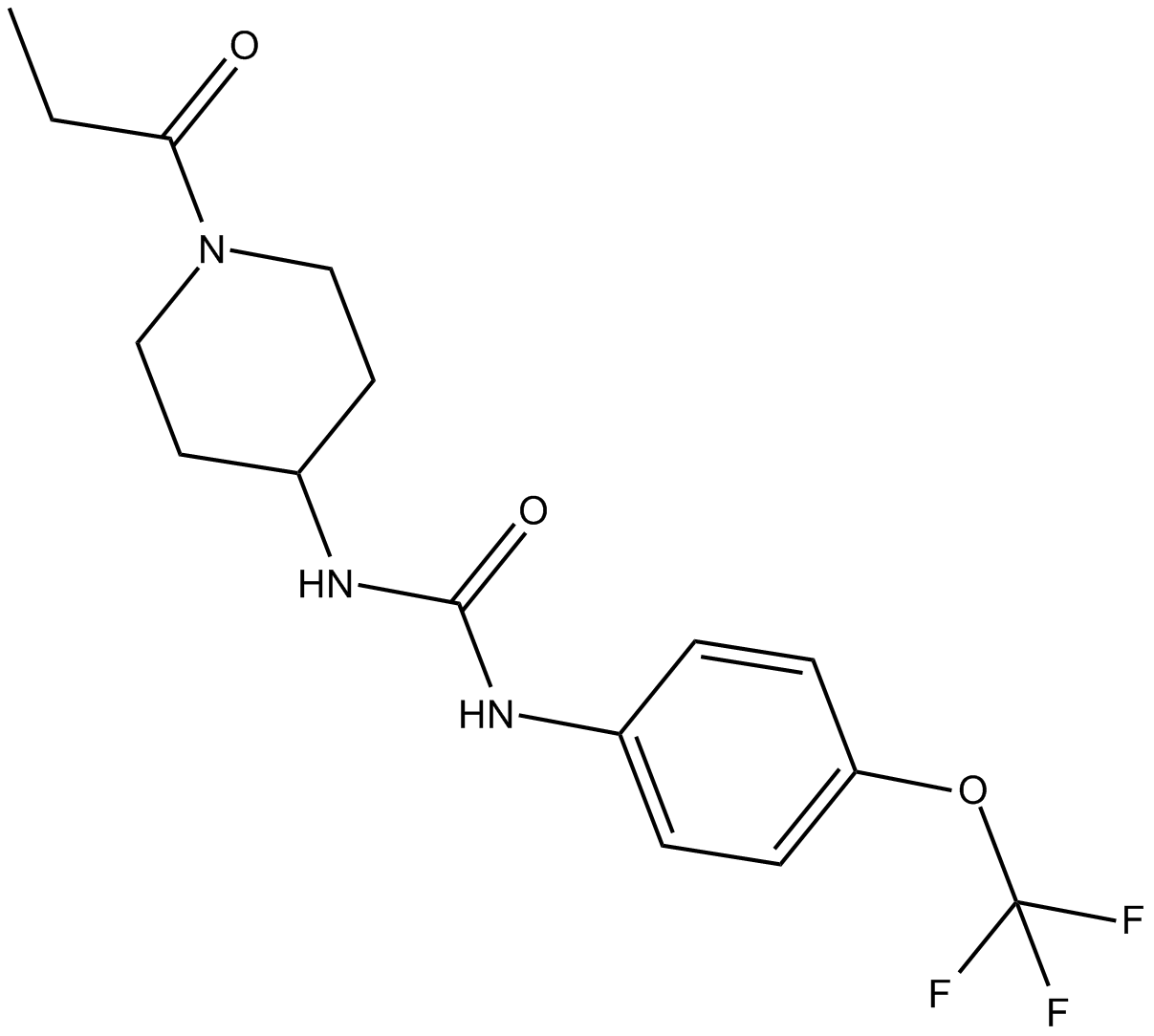
TPPU
IC50: human and mouse sEH (IC50 = 3.7 and 2.8 nM, respectively)
TPPU is a potent inhibitor of both human and mouse sEH.
The soluble epoxide hydrolase (sEH) can convert epoxides to the corresponding diols by the catalytic addition of a water molecule. sEH is implicated in various disease states for its ability to metabolize fatty acid epoxides such as epoxyeicosatrienoic acids and leukotoxin, important endogenous signaling lipids, to less active dihydroxyeicosatrienoic acids and toxic, proinflammatory leukotoxin diols, respectively. sEH inhibitors are of growing interest for therapeutic use since they have been shown to increase the in vivo concentration of EETs and other fatty acid epoxides.
In vitro: TPPU was identified as a potent inhibitor of both human and mouse sEH with IC50 of 3.7 and 2.8 nM, respectively [1].
In vivo: Animal study found that oral administration of 13 1-aryl-3-(1-acylpiperidin-4-yl)urea inhibitors including TPPU in mice showed substantial improvements in pharmacokinetic parameters over previously reported 1-adamantylurea based inhibitors. For example, 1-(1-(cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea, a close analog of TPPU, had a 7-fold increase in potency, a 65-fold increase in Cmax, and a 3300-fold increase in AUC over its adamantine analogue 1-(1-adamantyl)-3-(1-propionylpiperidin-4-yl)urea. This sEH inhibitor displayed a 1000-fold increase in potency when compared to morphine by reducing hyperalgesia using the in vivo carrageenan induced inflammatory pain model [1].
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] Rose, T. E.,Morisseau, C.,Liu, J.Y., et al. 1-aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010 Oct 14;53(19):7067-75.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 359.3 |
| Cas No. | 1222780-33-7 |
| Formula | C16H20F3N3O3 |
| Solubility | ≥120 mg/mL in DMSO; ≥54.8 mg/mL in EtOH; insoluble in H2O |
| Chemical Name | N-[1-(1-oxopropyl)-4-piperidinyl]-N’-[4-(trifluoromethoxy)phenyl)-urea |
| SDF | Download SDF |
| Canonical SMILES | CCC(N(CC1)CCC1NC(Nc(cc1)ccc1OC(F)(F)F)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure