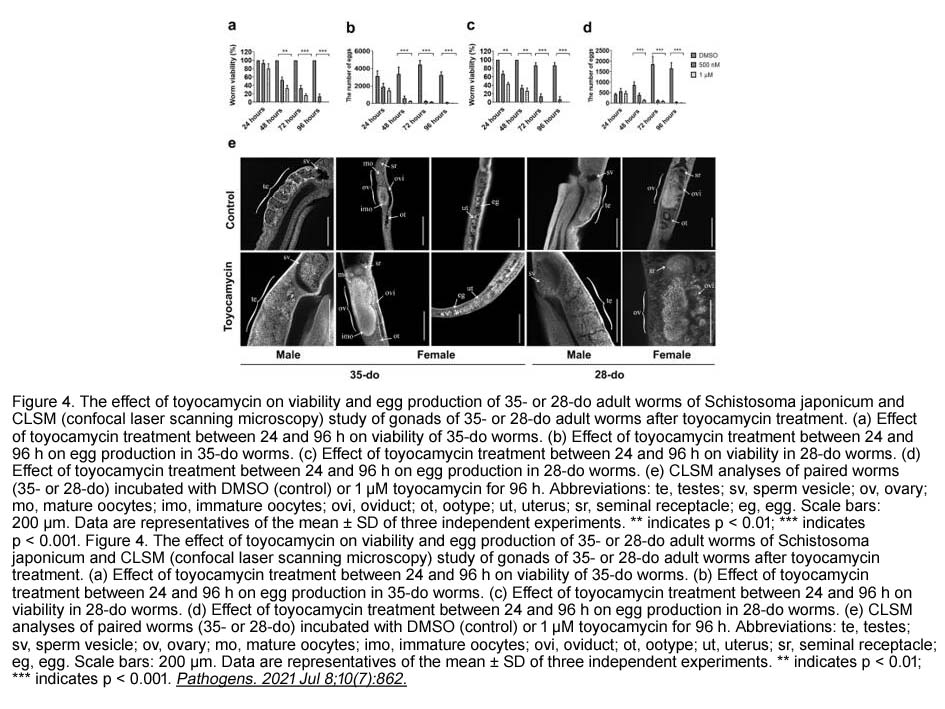
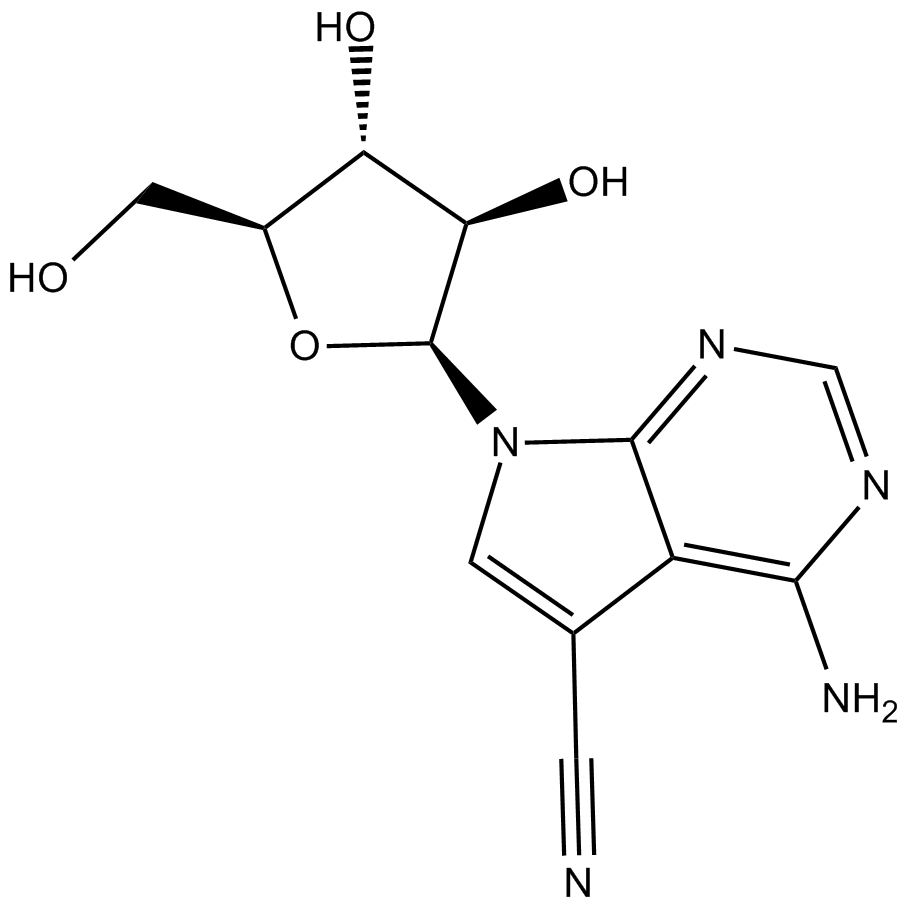
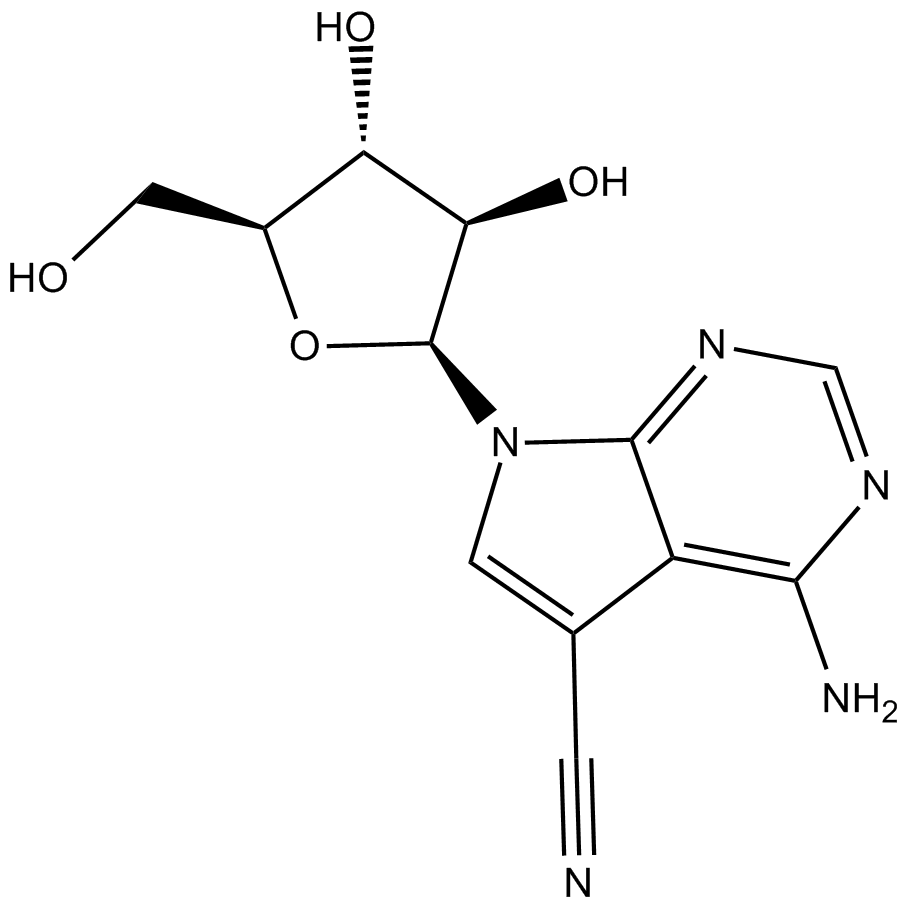
Toyocamycin
Toyocamycin is an inhibitor of phosphatidylinositol kinase. It is known as an antifungal antibiotic. [1]
Phosphatidylinositol kinase is one of the important enzymes that take part in the regulation of the pathway of phosphatidylinositol turnover. Phosphatidylinositol turnover is studied to be involved in the cellular response to mitogens and transformation.[1]
Toyocamycin can suppress thapsigargin-, tunicamycin- and 2-deoxyglucose-induced XBP1 mRNA splicing in HeLa cells. This suppression doesn’t affect the activating of transcription factor 6 (ATF6) and PKR-like ER kinase (PERK)’s activation. Toyocamycin prevents IRE1a-induced XBP1 mRNA cleavage in vitro. [2]
In mammalian cells, toyocamycin inhibits RNA synthesis. Toyocamycin induces apoptosis of MM cells including bortezomib-resistant cells at nanomolar levels in a dose-dependent manner. It also inhibited growth of xenografts in an in vivo model of human multiple myeloma. It is also a lead compound for developing anti-MM therapy and XBP1 as an appropriate molecular target for anti-multiple myeloma therapy.[2]
References:
[1]Nishioka H, Sawa T, etal. , Inhibition of phosphatidylinositol kinase by toyocamycin. J Antibiot (Tokyo). 1990 Dec;43(12):1586-9.
[2]Ri M, Tashiro E, Oikawa D, etal. , Identification of Toyocamycin,an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J. 2012 Jul;2(7):e79.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 291.26 |
| Cas No. | 606-58-6 |
| Formula | C12H13N5O4 |
| Solubility | ≥77 mg/mL in DMSO; ≥1.16 mg/mL in EtOH with gentle warming and ultrasonic; ≥1.34 mg/mL in H2O with gentle warming and ultrasonic |
| Chemical Name | 4-amino-7-((2S,3R,4R,5S)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile |
| SDF | Download SDF |
| Canonical SMILES | Nc1c(c(C#N)c[n]2[C@H]([C@@H]3O)O[C@@H](CO)[C@@H]3O)c2ncn1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
three MM cell lines, RPMI8226, XG7 and U266 |
|
Preparation method |
The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.01, 0.03 and 0.1 μM; 24 h |
|
Applications |
In RPMI8226 cells, treatment with 10 nM or higher concentrations of toyocamycin reduced the levels of spliced isoform of XBP1 protein and resulted in caspase activation. Toyocamycin also reduced the levels of spliced-XBP1 in two other MM cell lines XG7 and U266. Toyocamycin also inhibited thapsigargin-induced expression of spliced XBP1 protein. |
| Animal experiment [1]: | |
|
Animal models |
SCID mice bearing human MM RPMI8226 xenograft |
|
Dosage form |
intraperitoneal injection, 0.5mg/kg twice weekly or 1.0mg/kg once weekly for 2 weeks. |
|
Application |
In SCID mice bearing human MM RPMI8226 xenograft, Toyocamycin alone showed robust anti-tumor activity resulting in smaller tumor volumes compared with controls on day 15. The combination treatment of BTZ with toyocamycin showed a trend toward enhancing anti-tumor activity. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Ri M, Tashiro E, Oikawa D, et al, Identification of Toyocamycin,an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J. 2012 Jul;2(7):e79. | |
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data