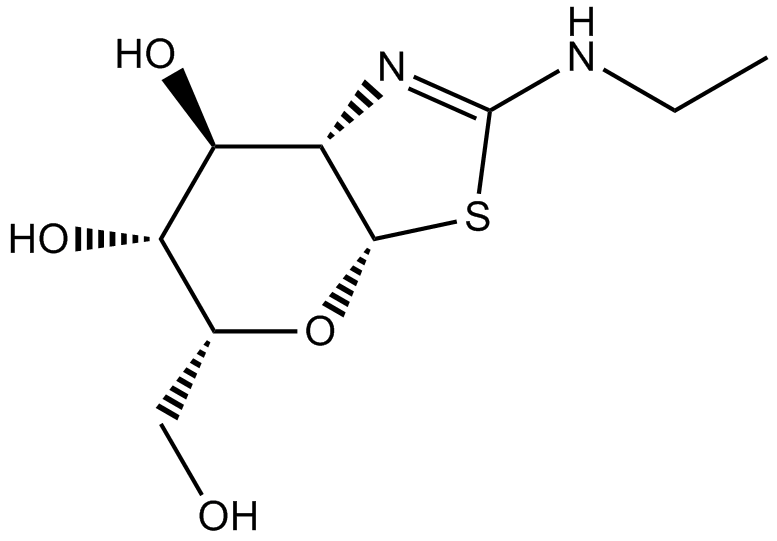
Thiamet G
Thiamet G is a potent and selective inhibitor of O-GlcNAcase with Ki value of 21 nM [1].
O-GlcNAcase is an enzyme that removes GlcNAc from proteins. O-GlcNAcylation refers to the posttranslational modification of O-linkage of N-acetyl-glucosamine moieties to threonine and serine residues on proteins [2].
Thiamet G is a potent and selective O-GlcNAcase inhibitor. In kinetic assays, thiamet G competitively inhibited human O-GlcNAcase with Ki value of 21 nM. Thiamet G was extremely stable in aqueous solution. In nerve growth factor (NGF)-differentiated PC-12 cells, thiamet G significantly increased cellular O-GlcNAc levels with EC50 value of 30 nM in a dose dependent way. Thiamet G significantly reduced phosphorylation levels at Ser396 and Thr231 of Tau by 2.1-fold and 2.7-fold, respectively. Also, thiamet G decreased the phosphorylation levels at Ser422 and Ser262 [1]. Thiamet G significantly sensitized human leukemia cell lines to paclitaxel, a microtubule-stabilizing agent [2].
In rats, thiamet G could readily cross the blood brain barrier and dose-dependently increased brain O-GlcNAc levels. Thiamet G reduced tau phosphorylation at Ser396, Thr231 and Ser422 by 2.7, 3.1 and 1.8-fold, respectively [1].
References:
[1]. Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol, 2008, 4(8): 483-490.
[2]. Ding N, Ping L, Shi Y, et al. Thiamet-G-mediated inhibition of O-GlcNAcase sensitizes human leukemia cells to microtubule-stabilizing agent paclitaxel. Biochem Biophys Res Commun, 2014, 453(3): 392-397.
- 1. Fei Yu, Xing Zhao, et al. "Regulation of T Cell Glycosylation by MXene/β‐TCP Nanocomposite for Enhanced Mandibular Bone Regeneration." Adv Healthc Mater. 2025 Jan 7:e2404015 PMID: 39764719
- 2. Chuwei Li, Zhang Qian, et al. "O-GlcNAc participates in the meiosis of aging oocytes by mediating mitochondrial function." Reproduction. 2024 Oct 1:REP-24-0138. PMID: 39405070
- 3. Chengjia You, Fangyuan Shen, et al. "O-GlcNAcylation mediates Wnt-stimulated bone formation by rewiring aerobic glycolysis." EMBO Rep. 2024 Sep 10. PMID: 39256595
- 4. Zhang Qian, Chuwei Li, et al. "Age-related elevation of O-GlcNAc causes meiotic arrest in male mice." Cell Death Discov. 2023 May 15;9(1):163. PMID: 37188682
- 5. Xuefei Y, Dongyan L, et al. "O-linkedN-acetylglucosamine affects mitochondrial homeostasis by regulating Parkin-dependent mitophagy in hyperoxia-injured alveolar type II cells injury." Respir Res 2023 Jan 16;24(1) PMID: 36647045
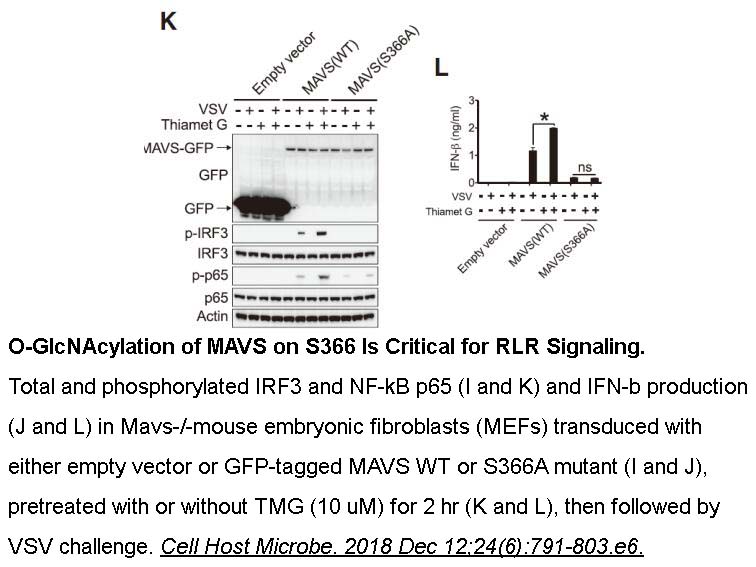
- 6. Li T, Li X, et al. "O-GlcNAc Transferase Links Glucose Metabolism to MAVS-Mediated Antiviral Innate Immunity." Cell Host Microbe. 2018 Dec 12;24(6):791-803.e6. PMID:30543776
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 248.3 |
| Cas No. | 1009816-48-1 |
| Formula | C9H16N2O4S |
| Solubility | ≥100 mg/mL in H2O; ≥12.4 mg/mL in DMSO; ≥2.64 mg/mL in EtOH with gentle warming and ultrasonic |
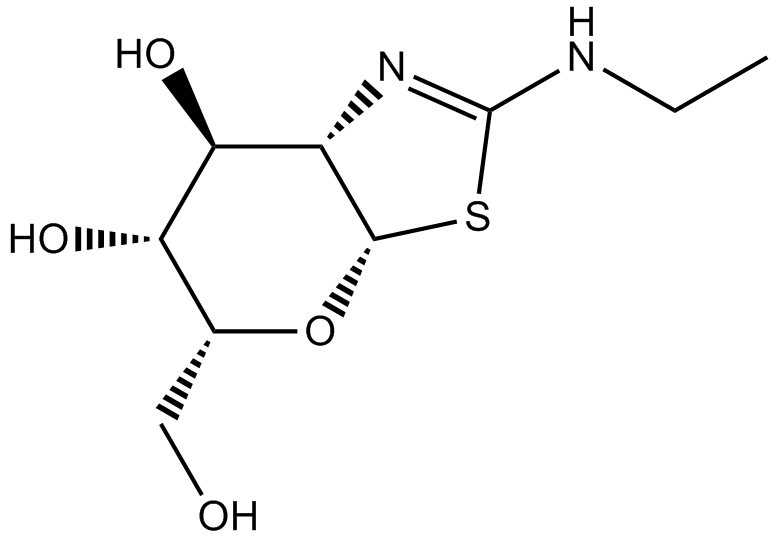
| Chemical Name | 2-(ethylamino)-5-(hydroxymethyl)-5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d][1,3]thiazole-6,7-diol |
| SDF | Download SDF |
| Canonical SMILES | CCNC(SC1OC(CO)C2O)=NC1C2O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1,2]: | |
|
Cell lines |
PC-12 cells, mesangial cells |
|
Preparation method |
The solubility of this compound in DMSO is > 12.4 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
1 nM to 250 mM, 24 h |
|
Applications |
Thiamet-G decreased phosphorylation of tau in PC-12 cells at pathologically relevant sites including Thr231 and Ser396. In PC-12 cells, treatment with thiamet-G for 24 h with concentrations ranging from 1 nM to 250 mM dose-dependently increased cellular O-GlcNAc levels. Thiamet G (12.5 nM and 25 nM) significantly enhanced p38 phosphorylation by increasing O-GlcNAcylation in mesangial cells. |
| Animal experiment [1,3]: | |
|
Animal models |
Male Sprague-Dawley rats, C57/bl mice |
|
Dosage form |
Intravenous injection, 50 mg/kg |
|
Application |
In rats, thiamet G (50 mg/kg, i.v.) crossed the blood brain barrier and then resulted in increase in brain O-GlcNAc levels in a dose- and time-dependent manner, and reduction of tau phosphorylation in the CA1 region of the hippocampus. O-GlcNAc accumulation induced by thiamet G stimulated chondrogenic differentiation in C57/bl mice by increasing the gene expression of differentiation markers, as well as the activity of MMP-2 and -9. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Yuzwa S A, Macauley M S, Heinonen J E, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo[J]. Nature chemical biology, 2008, 4(8): 483-490. [2]. Goldberg H, Whiteside C, Fantus I G. O-linked β-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells[J]. American Journal of Physiology-Endocrinology and Metabolism, 2011, 301(4): E713-E726. [3].Andrés-Bergós J, Tardio L, Larranaga-Vera A, et al. The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo[J]. Journal of Biological Chemistry, 2012, 287(40): 33615-33628. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data