TCEP hydrochloride
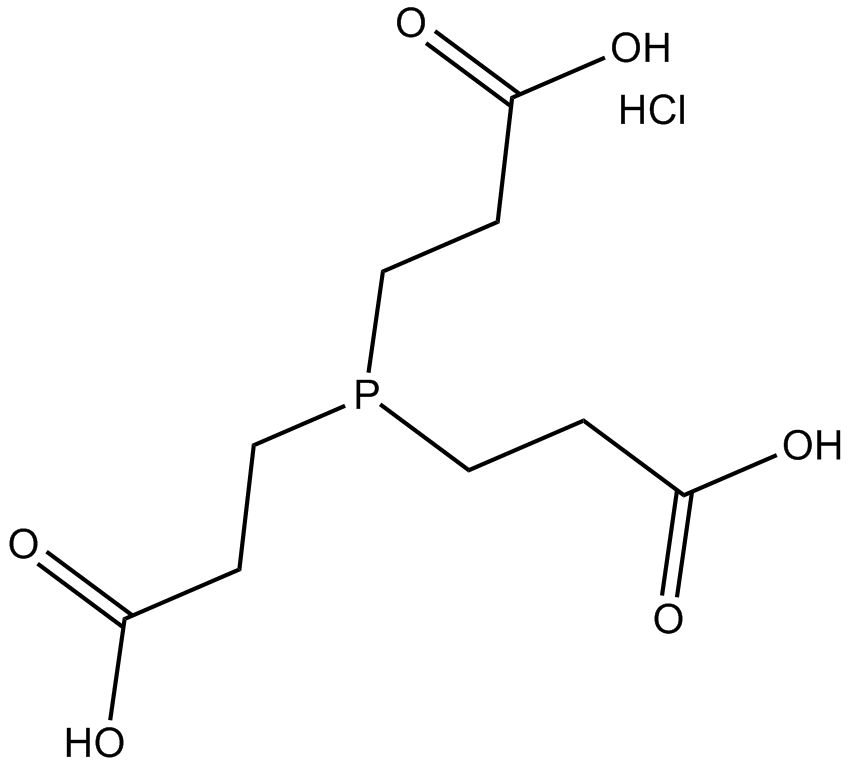
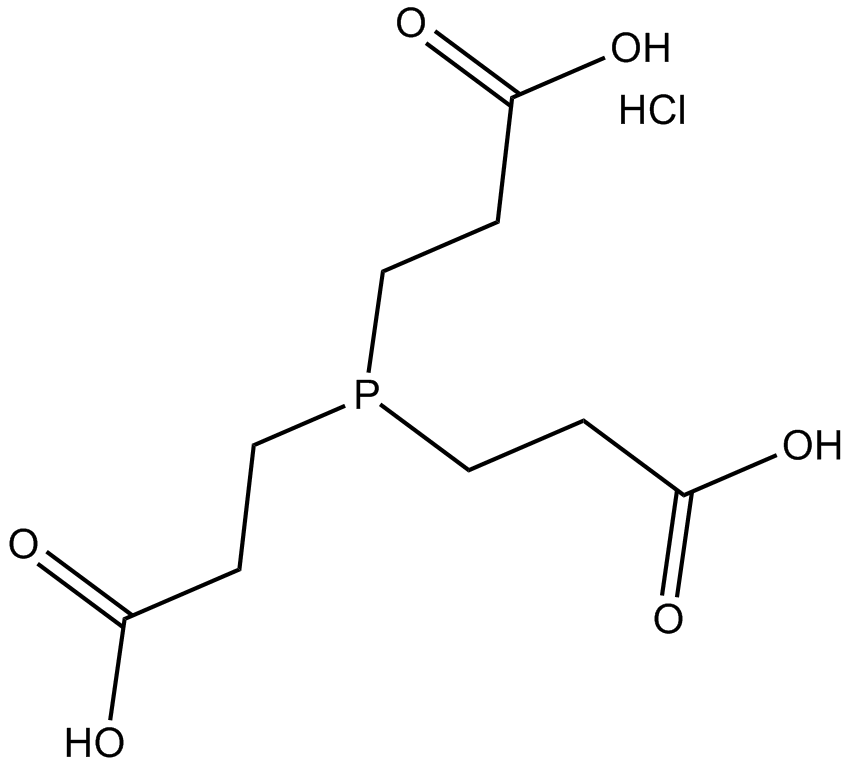
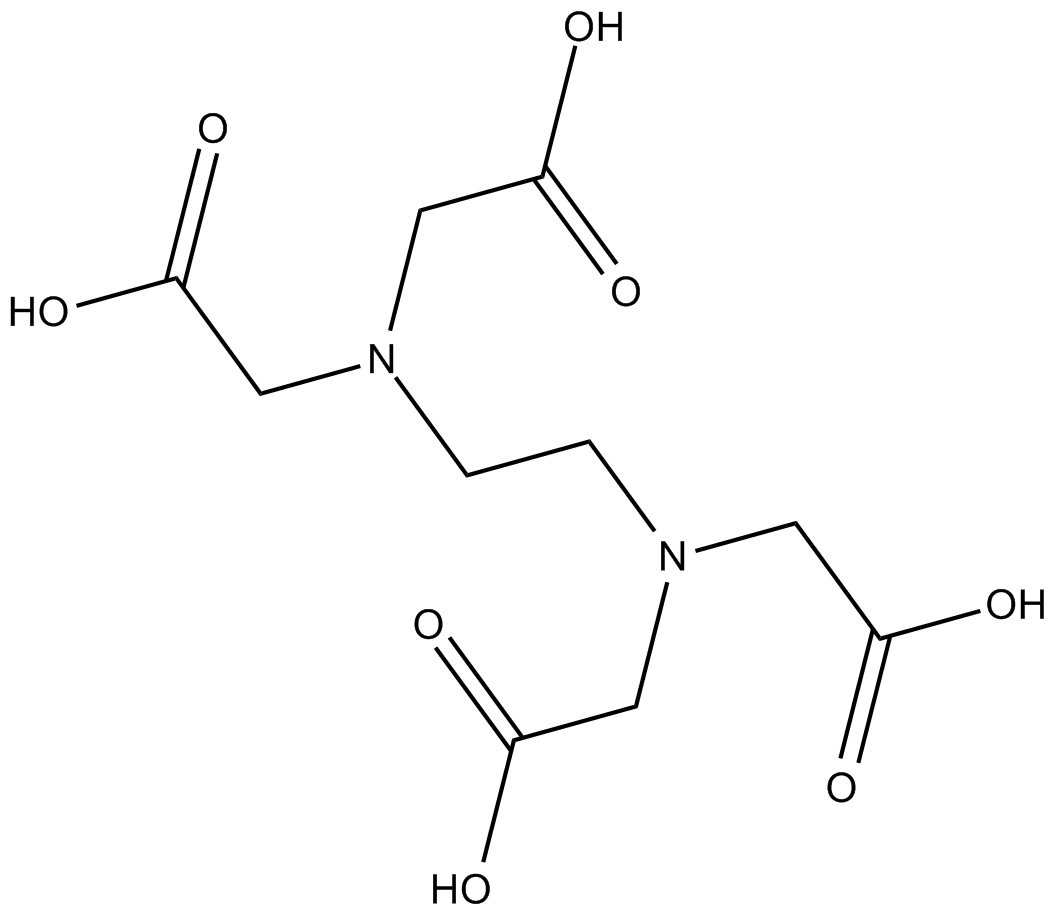
Tris(2-carboxyethyl) phosphine hydrochloride (TCEP hydrochloride, CAS 51805-45-9) is a water-soluble reducing agent widely employed in biochemical research. As a non-volatile, thiol-free compound, it cleaves disulfide bonds via a selective reduction reaction, converting them into free thiols. TCEP hydrochloride facilitates the reduction of various functional groups, including azides, sulfonyl chlorides, nitroxides, and dimethyl sulfoxide derivatives, thus serving as a versatile reagent in organic synthesis. In biological assays, this reagent allows the complete reduction of dehydroascorbic acid (DHA) to ascorbic acid under acidic conditions within samples. It is commonly used in combination with proteolytic enzymes to support protein digestion and hydrogen-deuterium exchange analyses.
Reference:
1. Sato Y, Uchiki T, Iwama M, Kishimoto Y et al. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol Pharm Bull. 2010;33(3):364-9.
2. Zhang HM, McLoughlin SM, Frausto SD et al. Simultaneous reduction and digestion of proteins with disulfide bonds for hydrogen/deuterium exchange monitored by mass spectrometry. Anal Chem. 2010 Feb 15;82(4):1450-4.
- 1. Chapman Ho, Clíona McMahon, et al. "Triggered ‘capture-and-release’ enables a high-affinity re-binding strategy for sensitivity enhancement in lateral flow assays." Chem Rxiv. 10 January 2025, Version 1
- 2. Wei Song, Yichen Zhao, et al. "The dual ubiquitin binding mode of SPRTN secures rapid spatiotemporal proteolysis of DNA-protein crosslinks." bioRxiv. November 26, 2024.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 286.65 |
| Cas No. | 51805-45-9 |
| Formula | C9H16ClO6P |
| Solubility | ≥28.7 mg/mL in H2O; insoluble in EtOH; ≥25.7 mg/mL in DMSO |
| Chemical Name | 3,3',3''-phosphinetriyltripropanoic acid hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | OC(CCP(CCC(O)=O)CCC(O)=O)=O.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure