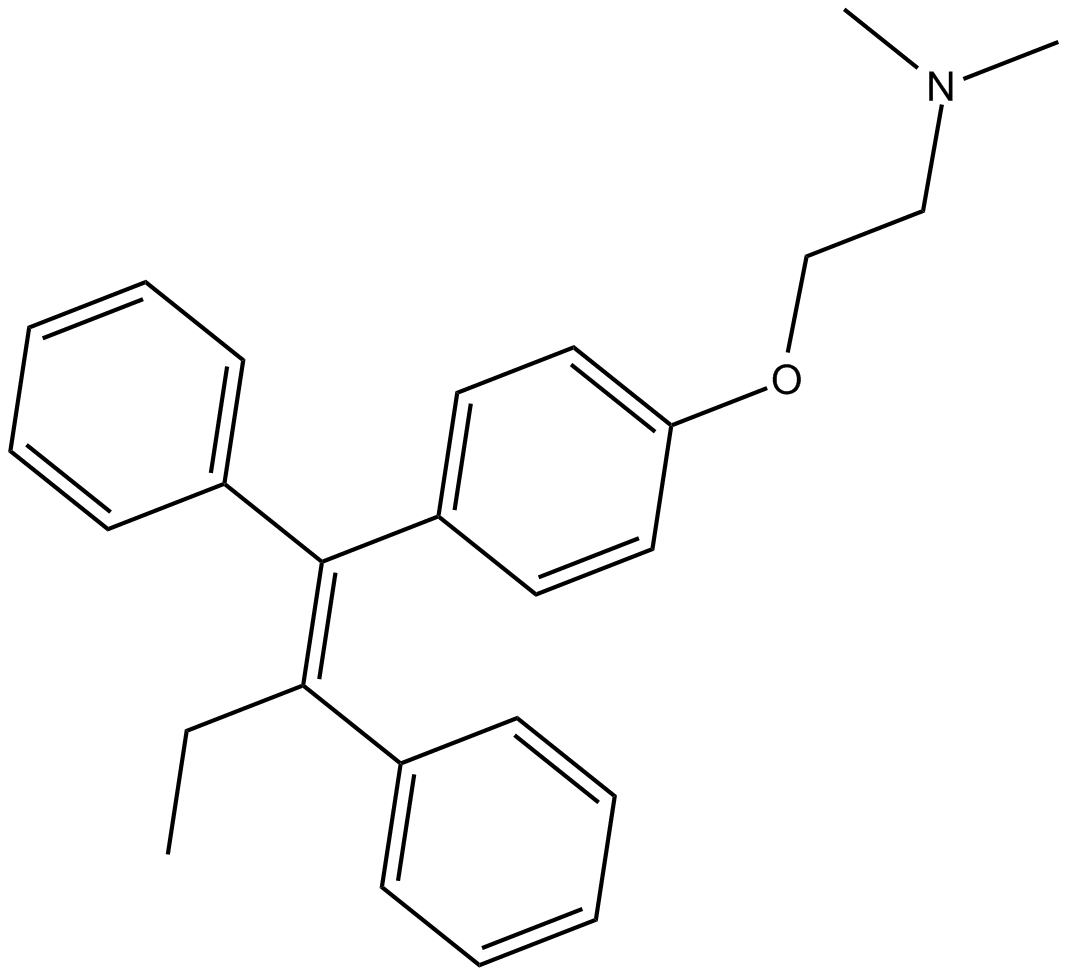
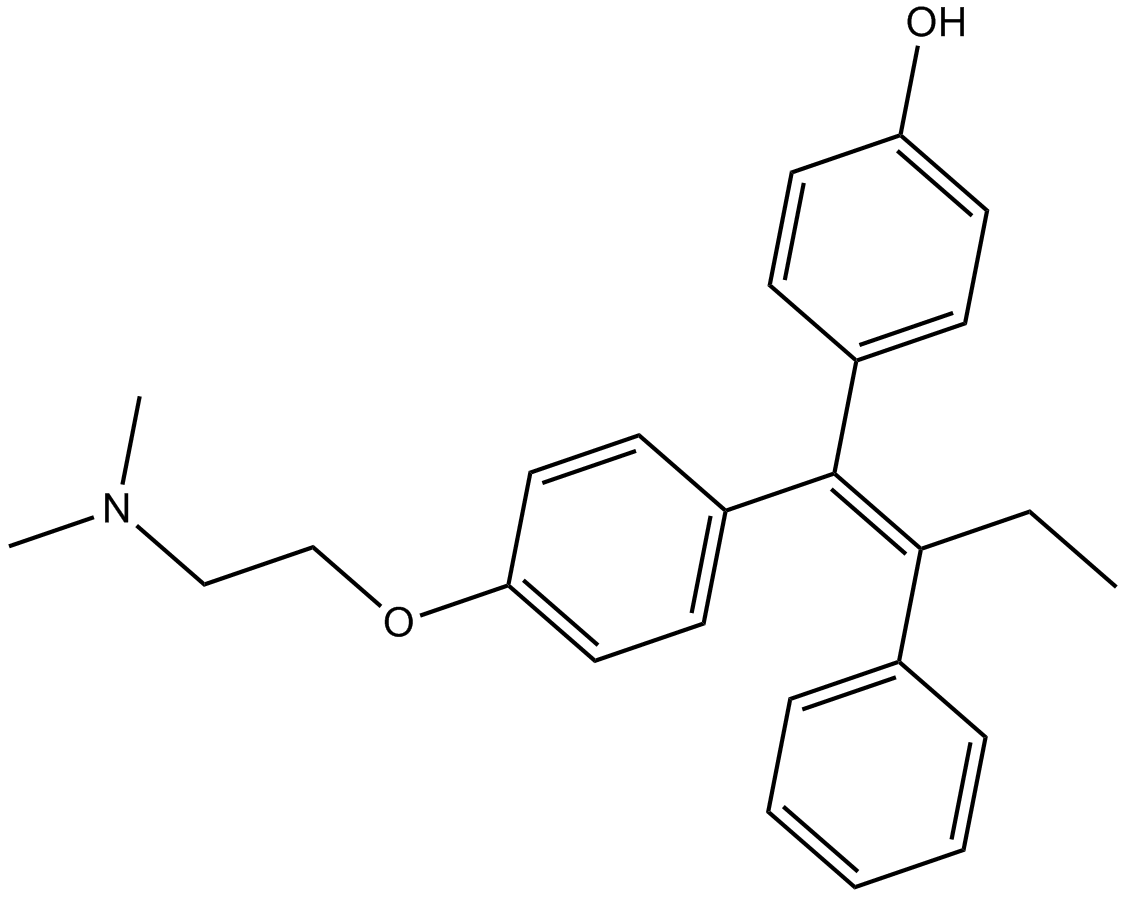
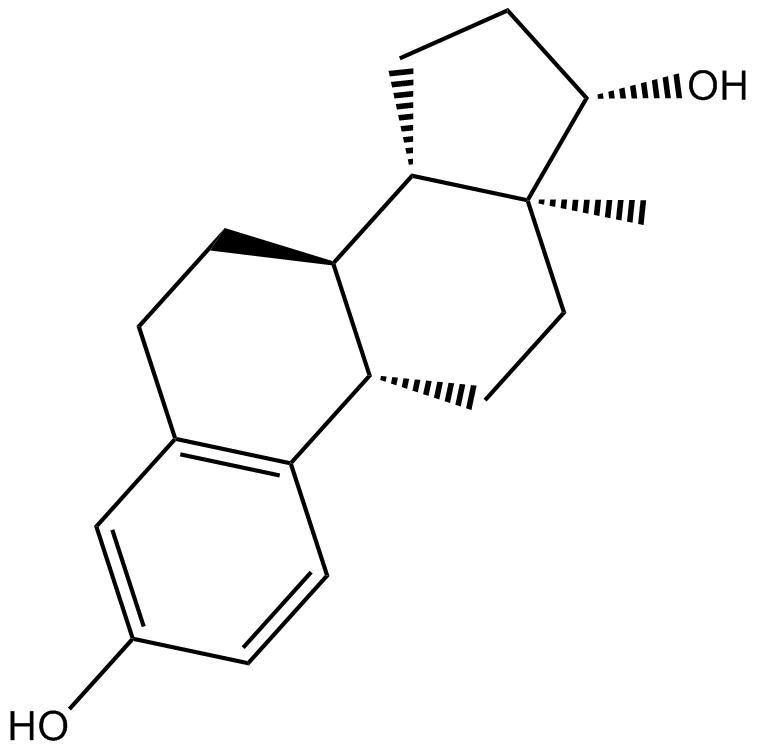
Tamoxifen
- 1. Hong Dong, Chenxi Liang, et al. "O-GlcNAc transferase plays dual antiviral roles by integrating innate immunity and lipid metabolism." Nat Commun. 2025 Aug 19;16(1):7721. doi: 10.1038/s41467-025-63085-y PMID: 40830102
- 2. Qiannan Ma, Ella X. Segal, et al. "Wnt Activation in Mature Dermal Adipocytes Leads to Lipodystrophy and Skin Fibrosis via ATGL‐Dependent Lipolysis." FASEB J. 2025 Jul 15;39(13):e70830 PMID: 40632520
- 3. Feng Lan, Jizhou Li, et al. "GZMK-expressing CD8+ T cells promote recurrent airway inflammatory diseases." Nature. 2025 Jan 15 PMID: 39814882
- 4. Qiannan Ma, Ella X. Segal, et al. "Wnt activation in mature dermal adipocytes leads to lipodystrophy and skin fibrosis via ATGL-dependent lipolysis." bioRxiv. November 18, 2024.
- 5. Sung Hoon Cho, Marissa A Jones, et al. "B cell expression of the enzyme PexRAP, an intermediary in ether lipid biosynthesis, promotes antibody responses and germinal center size." bioRxiv. 2024 Oct 26:2024.10.17.618760 PMID: 39464149
- 6. Nicolás Marichal, Sophie Péron, et al. "Reprogramming astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site–deficient Ascl1." Sci Adv. 2024 Oct 25;10(43):eadl5935. PMID: 39454007
- 7. Junrong Cai, Yuping Quan, et al. "The browning and mobilization of subcutaneous white adipose tissue supports efficient skin repair." Cell Metab. 2024 Jun 4;36(6):1287-1301.e7. PMID: 38838641
- 8. Bing-Ling Peng, Ting Ran, et al. "A CARM1 Inhibitor Potently Suppresses Breast Cancer Both In Vitro and In Vivo." J Med Chem. 2024 May 23;67(10):7921-7934. PMID: 38713486
- 9. Tyler J Dause, Jiyeon K Denninger, et al. "Autocrine VEGF drives neural stem cell proximity to the adult hippocampus vascular niche." Life Sci Alliance. 2024 Apr 17;7(7):e202402659. PMID: 38631901
- 10. Yasufumi Katanasaka, Harumi Yabe, et al. "Fibroblast-specific PRMT5 deficiency suppresses cardiac fibrosis and left ventricular dysfunction in male mice." Nat Commun. 2024 Mar 19;15(1):2472. PMID: 38503742
- 11. Nicolás Marichal, Sophie Péron, et al. "Reprogramming early cortical astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site deficient Ascl1." bioRxiv. November 04, 2023
- 12. Ming Wu, Dongping Chen, et al. "Injury induced renal fibrosis promotes cystogenesis and cyst growth in adult mice with autosomal dominant polycystic kidney disease." bioRxiv. October 10, 2023
- 13. Dinh Duc Nguyen, Eugene Kim, et al. "Deficiency in mammalian STN1 promotes colon cancer development via inhibiting DNA repair." Sci Adv. 2023 May 10;9(19):eadd8023. PMID: 37163605
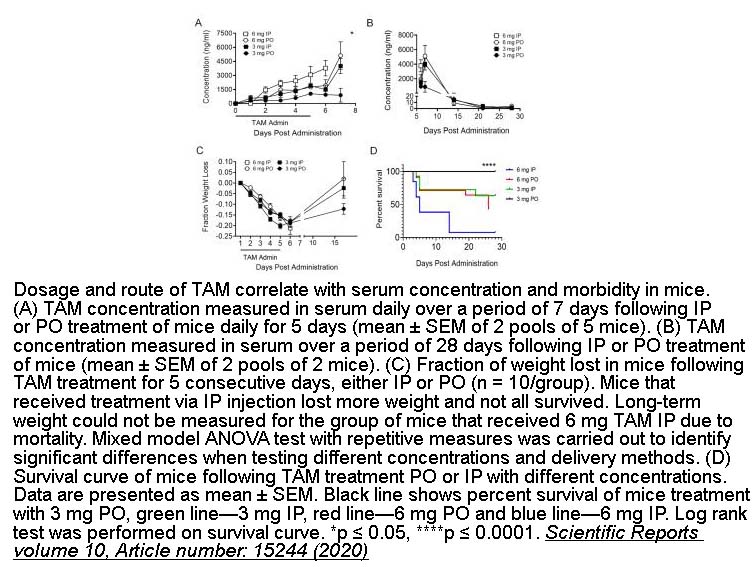
- 14. Rachel S. Donocoff, Nato Teteloshvili, et al. "Optimization of tamoxifen-induced Cre activity and its effect on immune cell populations." Scientific Reports volume 10, Article number: 15244 (2020). PMID:32943672
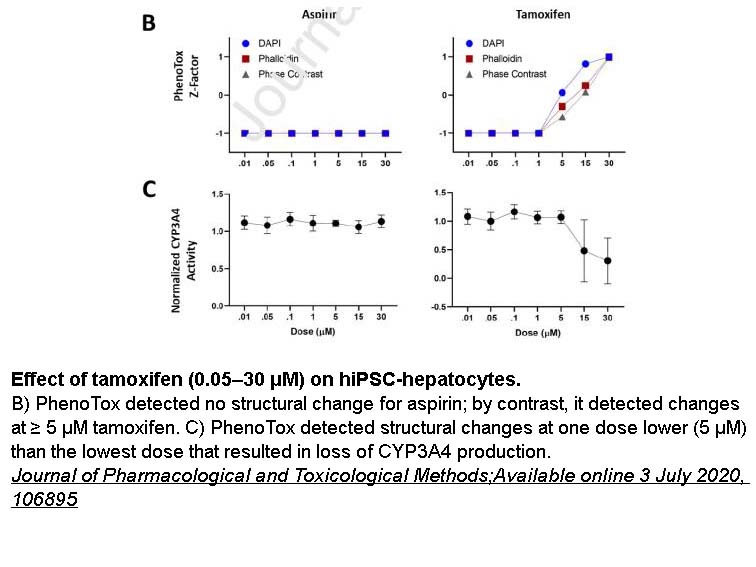
- 15. Mahnaz Maddah, Mohammad A Mandegar, et al. "Quantifying drug-induced structural toxicity in hepatocytes and cardiomyocytes derived from hiPSCs using a deep learning method." J Pharmacol Toxicol Methods. 2020 Sep;105:106895. PMID:32629158
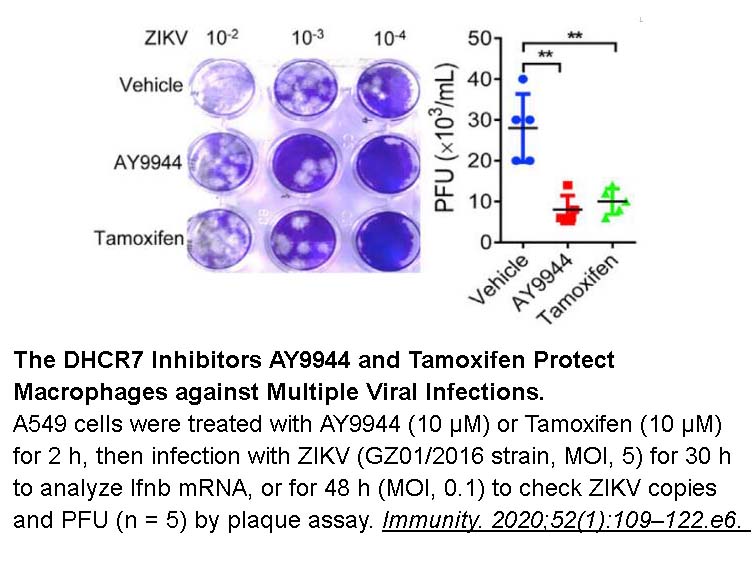
- 16. Xiao J, Li W, et al. "Targeting 7-Dehydrocholesterol Reductase Integrates Cholesterol Metabolism and IRF3 Activation to Eliminate Infection." Immunity. 2020;52(1):109–122.e6. PMID:31882361
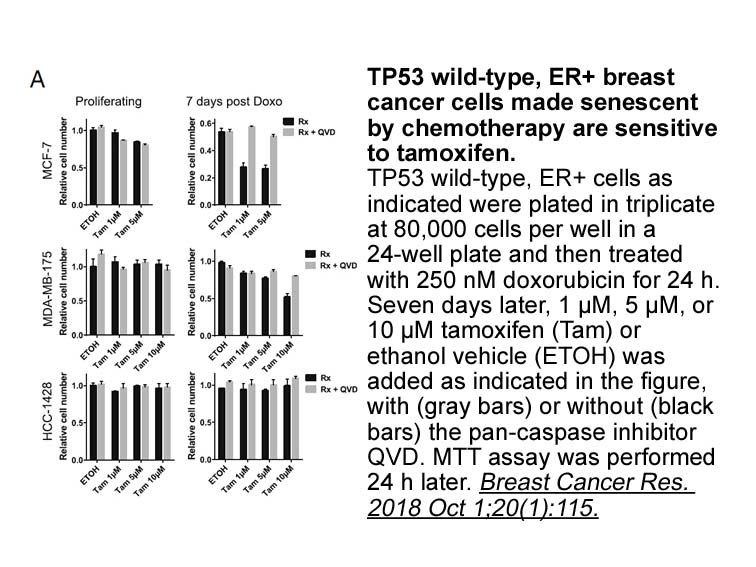
- 17. Ungerleider NA, Rao SG, et al. "Breast cancer survival predicted by TP53 mutation status differs markedly depending on treatment." Breast Cancer Res. 2018 Oct 1;20(1):115. PMID:30285883
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 371.51 |
| Cas No. | 10540-29-1 |
| Formula | C26H29NO |
| Synonyms | ICI 47699; (Z)-Tamoxifen; trans-Tamoxifen |
| Solubility | ≥18.6 mg/mL in DMSO; insoluble in H2O; ≥85.9 mg/mL in EtOH |
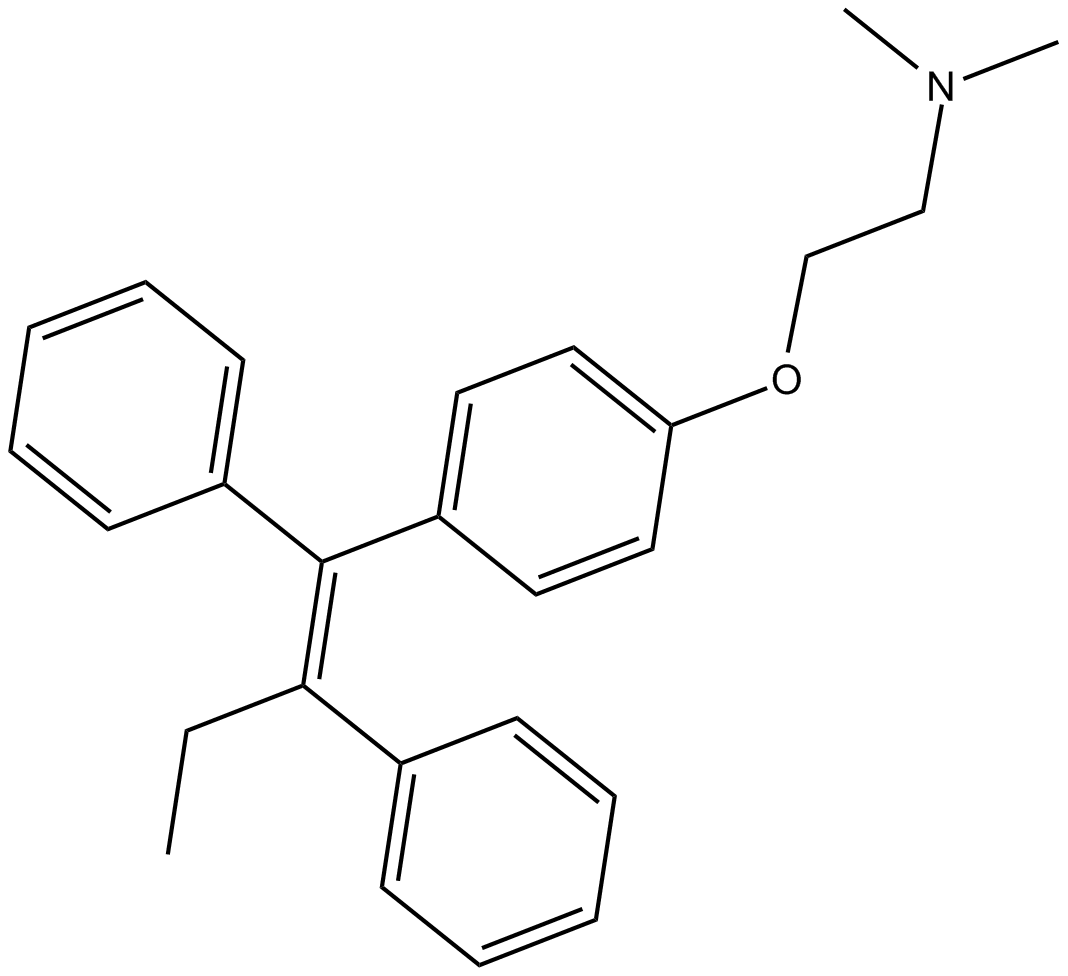
| Chemical Name | 2-[4-[(1Z)-1,2-diphenyl-1-buten-1-yl]phenoxy]-N,N-dimethyl-ethanamine |
| SDF | Download SDF |
| Canonical SMILES | CC/C(\c1ccccc1)=C(\c1ccccc1)/c(cc1)ccc1OCCN(C)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
PC3 and PC3-M prostate carcinoma cells, DU-145 cells |
|
Preparation method |
The solubility of this compound in DMSO is >18.6mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
10 μM, 3 days |
|
Applications |
In PC3-M cells, treatment with tamoxifen for 3days dose-dependently inhibited cell growth. Tamoxifen (10 μM) inhibited protein kinase C activity in PC3-M cells. Tamoxifen and TGF-β showed additive effects upon thymidine uptake in PC3-M cells. Cytosolic Rb protein decreased 12 hr after treatment with tamoxifen, continuing to decline for at least 24 hr. In the nucleus, the phosphorylated form of Rb disappeared between 12–24 hr after treatment with tamoxifen. |
| Animal experiment [2]: | |
|
Animal models |
Ovariectomized nude mice bearing MCF-7 xenografts |
|
Dosage form |
21 days |
|
Application |
Treatment with TAM resulted in a slowing of tumor growth (tumor doubling time, 12 days), a significant increase in Tpot to 6.6 days, and a decrease in %LI to 8% by 23 days posttreatment. TAM treatment significantly decreased tumor cell proliferation in MCF-7 xenografts. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Rohlff C, Blagosklonny M V, Kyle E, et al. Prostate cancer cell growth inhibition by tamoxifen is associated with inhibition of protein kinase C and induction of p21waf1/cip1[J]. The Prostate, 1998, 37(1): 51-59. [2]. Sarkaria J N, Gibson D F C, Jordan V C, et al. Tamoxifen-induced increase in the potential doubling time of MCF-7 xenografts as determined by bromodeoxyuridine labeling and flow cytometry[J]. Cancer research, 1993, 53(18): 4413-4417. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data