Ubiquitination/ Proteasome
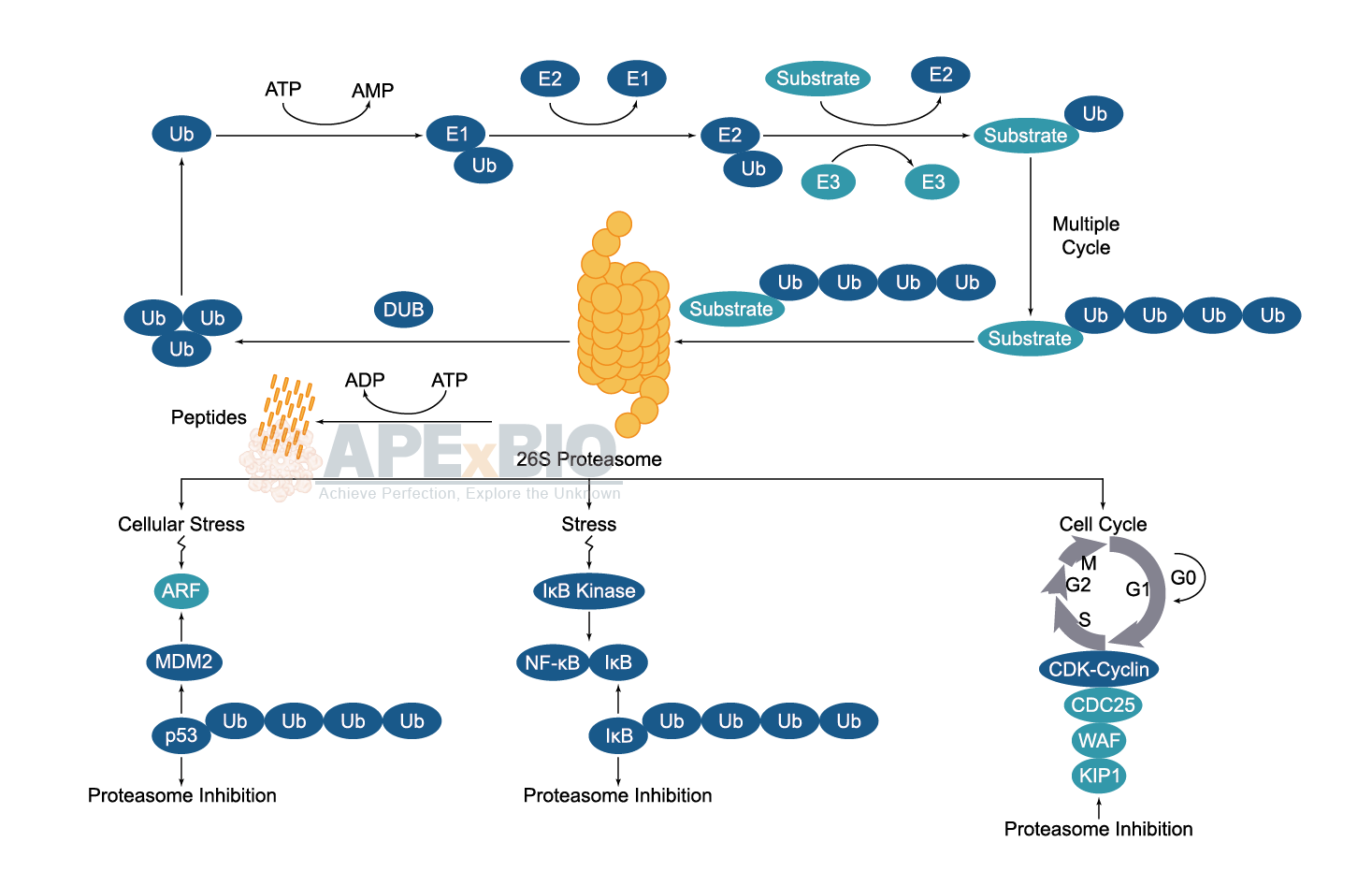
Once the substrate protein is labeled, proteasome will bind to a polyubiquitin chain, allowing the degradation of the labeled protein. The polyubiquitinated target protein is then recognized and degraded by the 26S proteasome. Deubiquitinating enzymes (DUBs) reverse the process of ubiquitination by removing ubiquitin from its substrate protein. Dysregulation of the ubiquitin-proteasome system has been linked to cancer, diabetes, cardiovascular and neurodegenerative diseases etc.
-
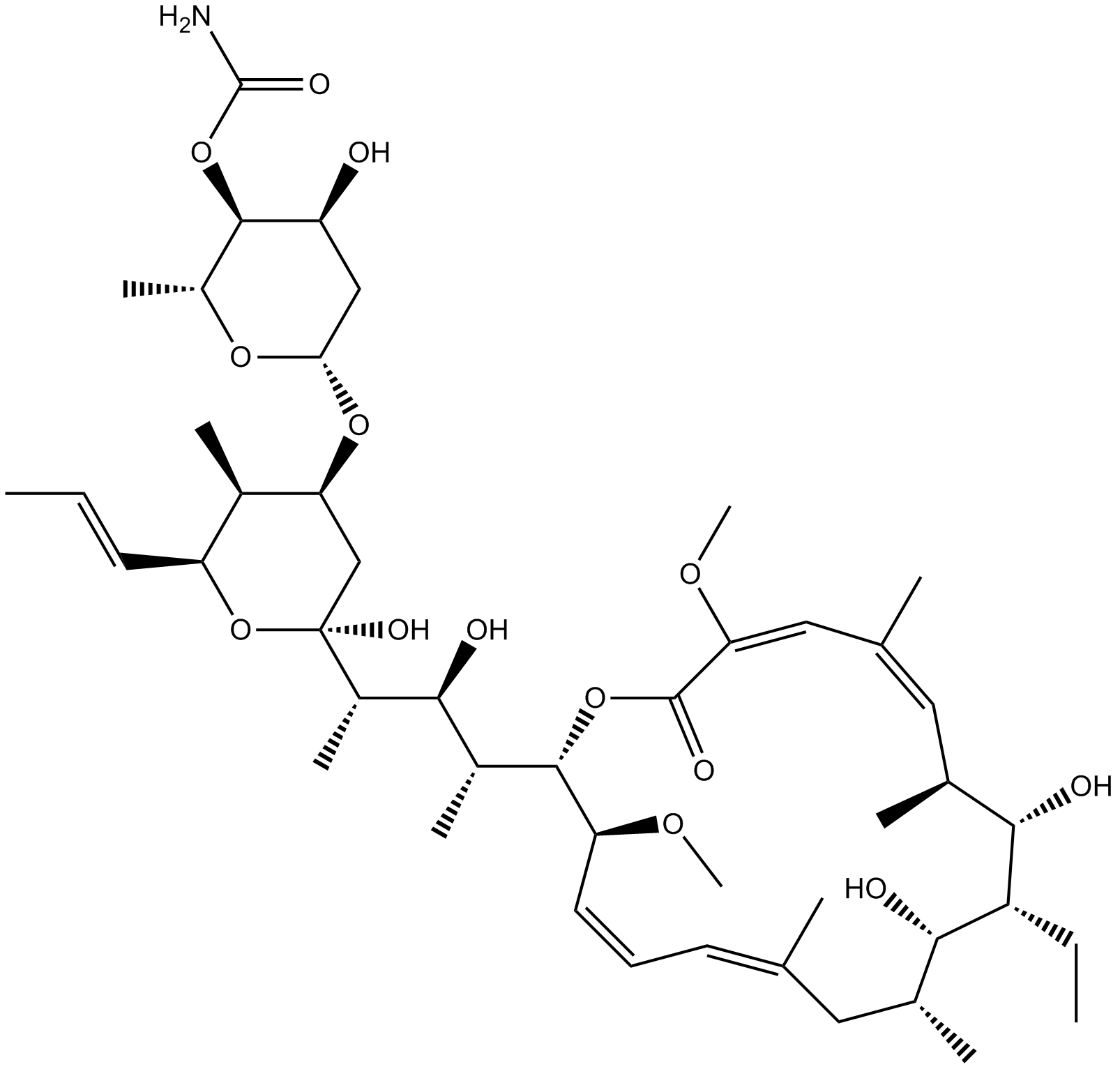 A8633 Concanamycin A1 CitationTarget: Vacuolar-type ATPaseSummary: V-type (vacuolar) H+-ATPase inhibitor
A8633 Concanamycin A1 CitationTarget: Vacuolar-type ATPaseSummary: V-type (vacuolar) H+-ATPase inhibitor -
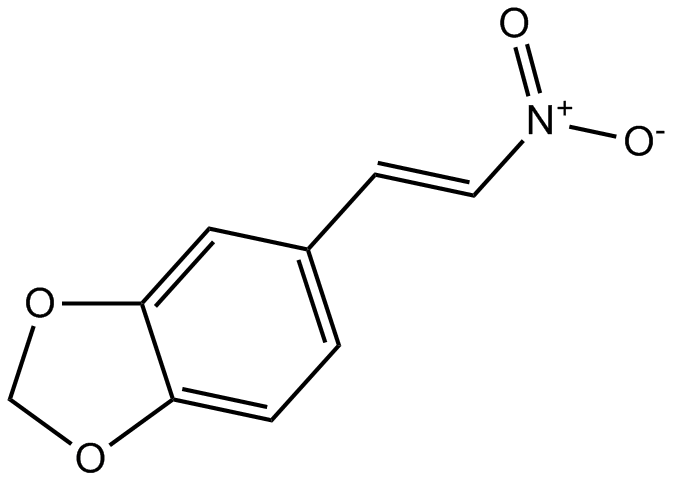 A8661 MNSSummary: Inhibitor of Src/Syk tyrosine kinases
A8661 MNSSummary: Inhibitor of Src/Syk tyrosine kinases -
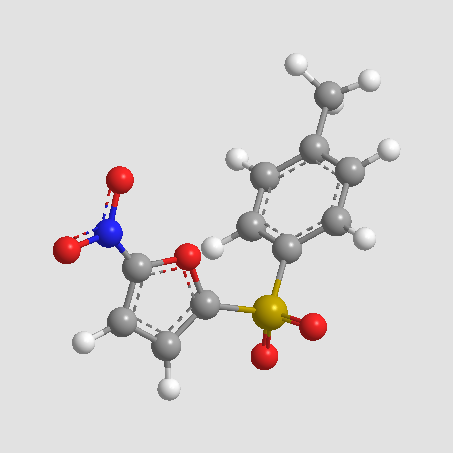 A8813 NSC697923Target: Ub-conjugating enzyme (E2) complexSummary: Inhibitor of E2 complex Ubc13-Uev1A,cell permeable and selective
A8813 NSC697923Target: Ub-conjugating enzyme (E2) complexSummary: Inhibitor of E2 complex Ubc13-Uev1A,cell permeable and selective -
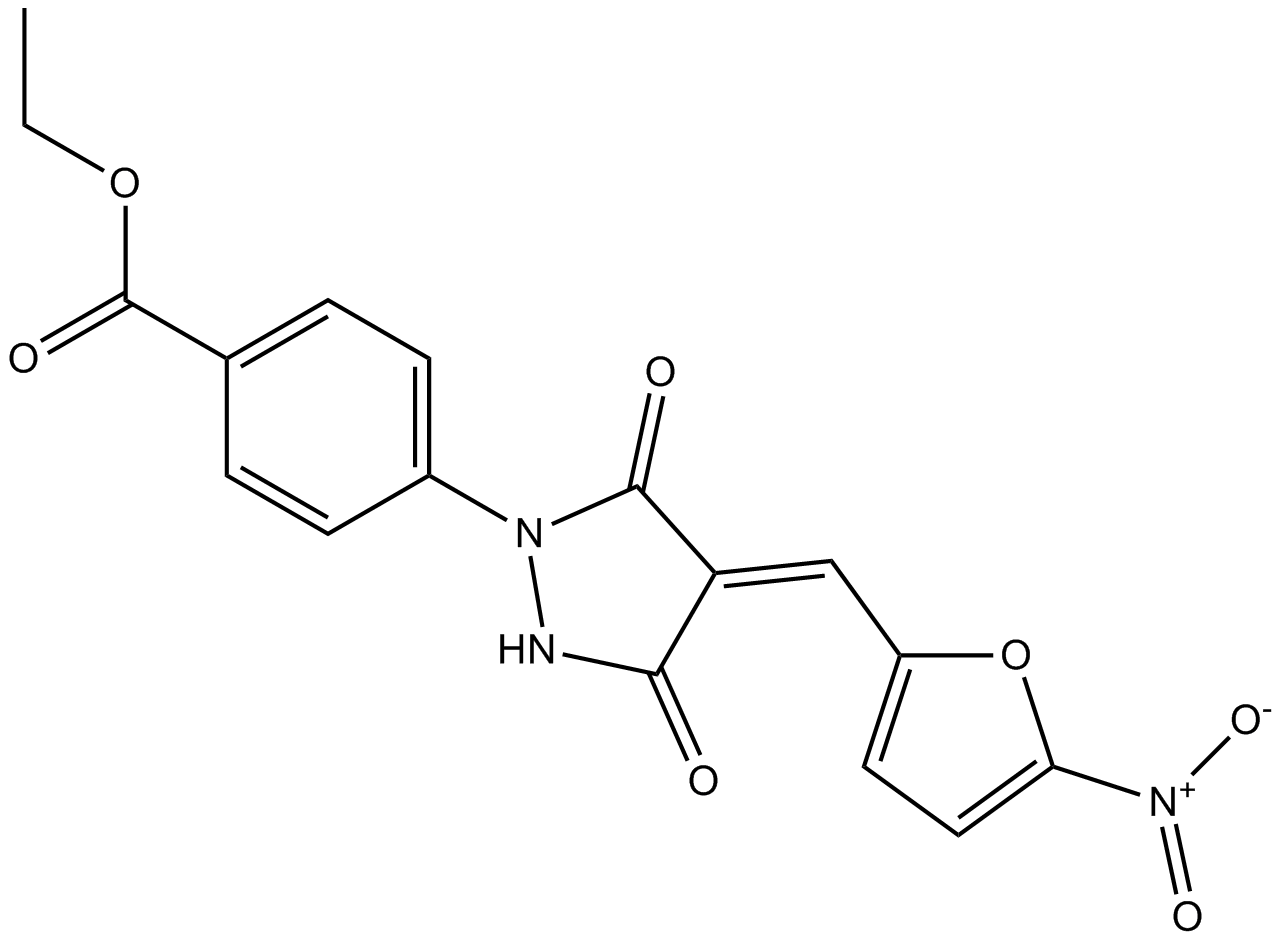 B1492 PYR-412 CitationTarget: ProteasomeSummary: inhibitor of Ubiquitin-Activating Enzyme (E1)
B1492 PYR-412 CitationTarget: ProteasomeSummary: inhibitor of Ubiquitin-Activating Enzyme (E1) -
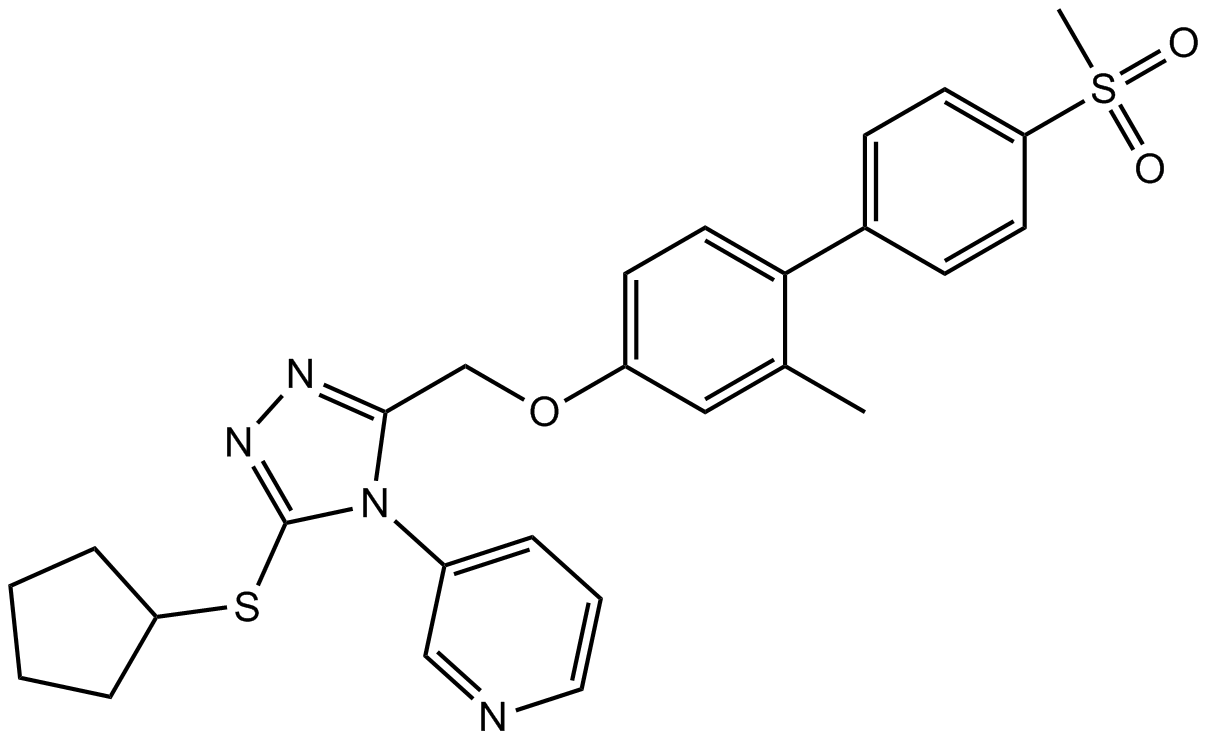 B2168 NMS-8733 CitationTarget: Valosin-containing protein (VCP)Summary: VCP/p97 inhibitor,selective and allosteric
B2168 NMS-8733 CitationTarget: Valosin-containing protein (VCP)Summary: VCP/p97 inhibitor,selective and allosteric -
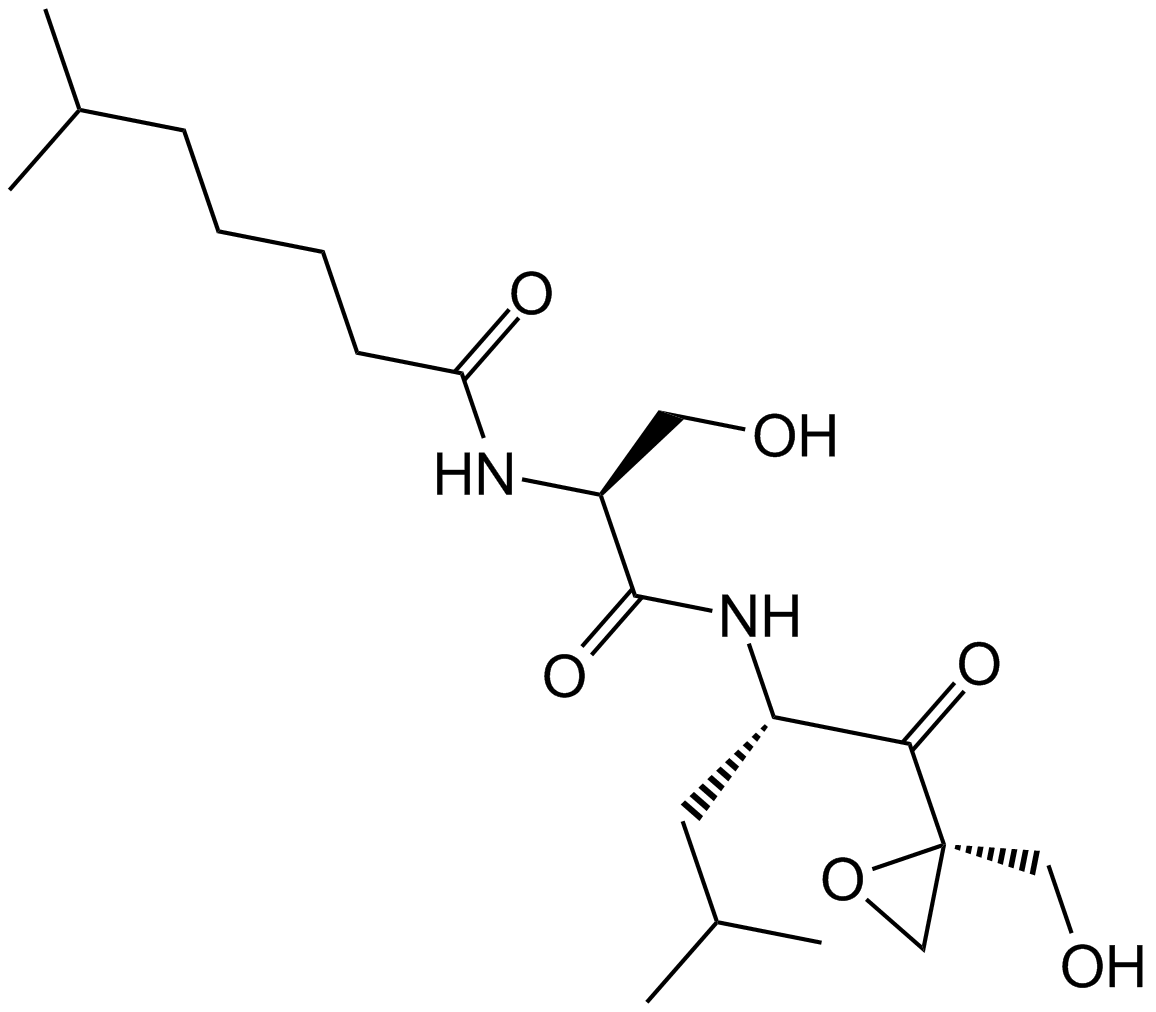 A8172 Dihydroeponemycin1 CitationSummary: Proteasome inhibitor,antitumor reagent,eponemycin ddrivative
A8172 Dihydroeponemycin1 CitationSummary: Proteasome inhibitor,antitumor reagent,eponemycin ddrivative -
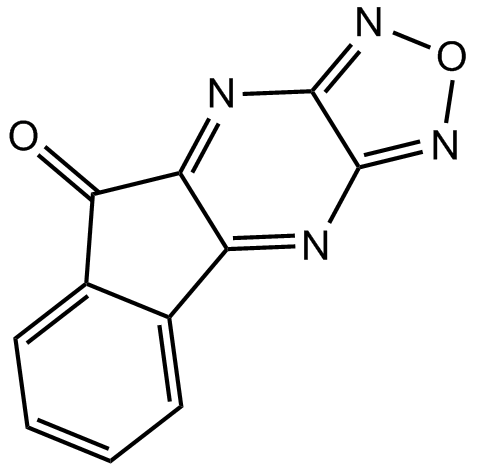 B5567 SMER 3Summary: yeast SCF family E3 ubiquitin ligase (SCFMet30) inhibitor
B5567 SMER 3Summary: yeast SCF family E3 ubiquitin ligase (SCFMet30) inhibitor -
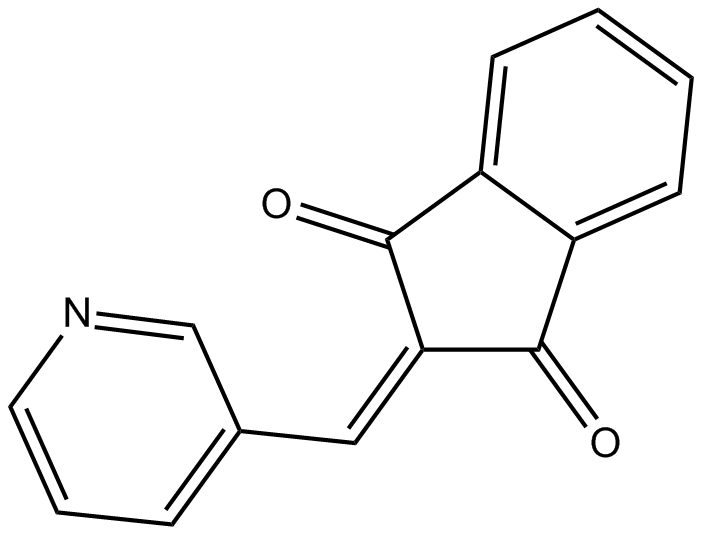 B5797 PRT 4165Summary: Bmi1/Ring1A-mediated ubiquitination inhibitor
B5797 PRT 4165Summary: Bmi1/Ring1A-mediated ubiquitination inhibitor -
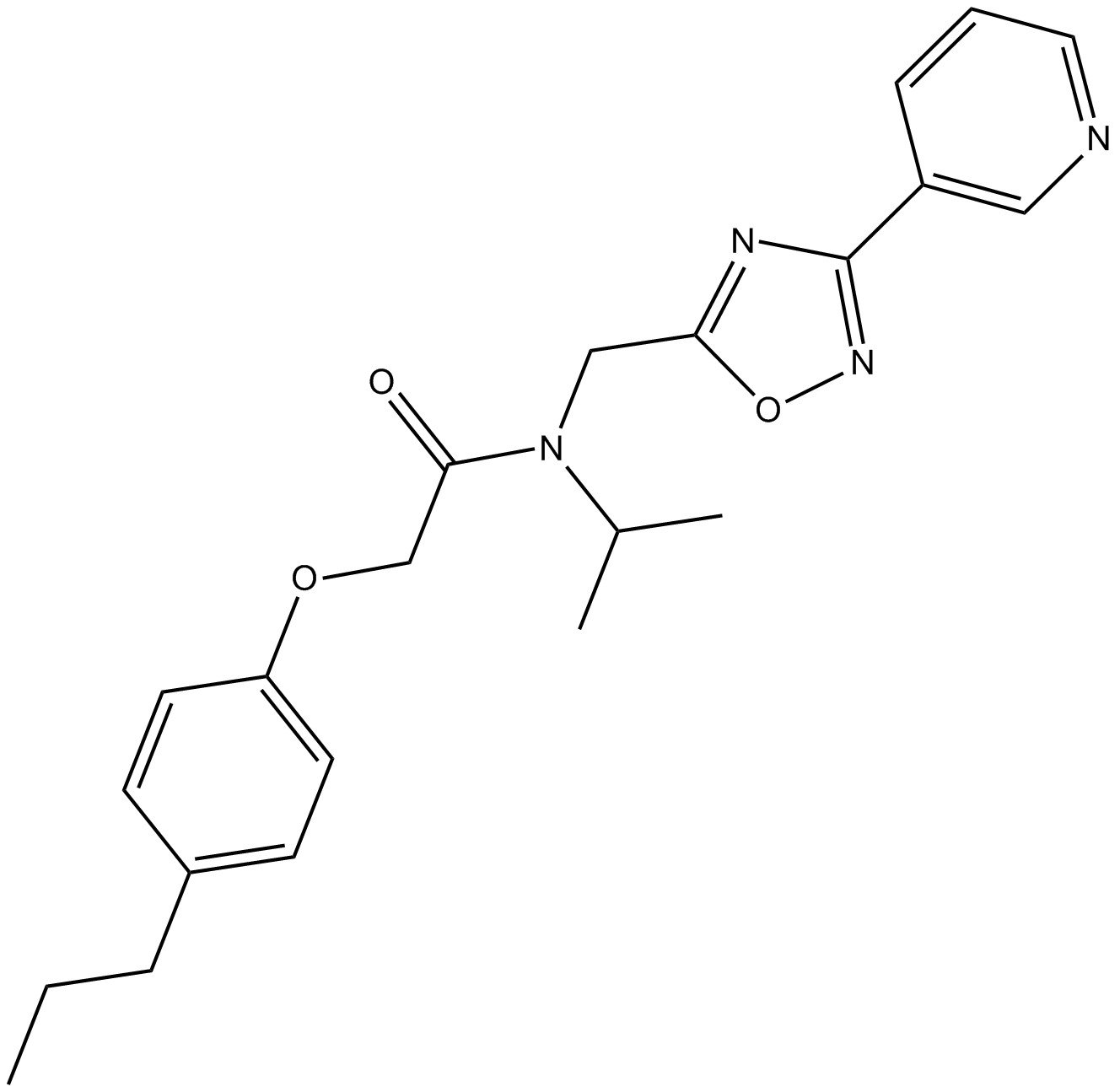 B4755 PI-1840Summary: Chymotrypsin-like (CT-L) inhibitor
B4755 PI-1840Summary: Chymotrypsin-like (CT-L) inhibitor -
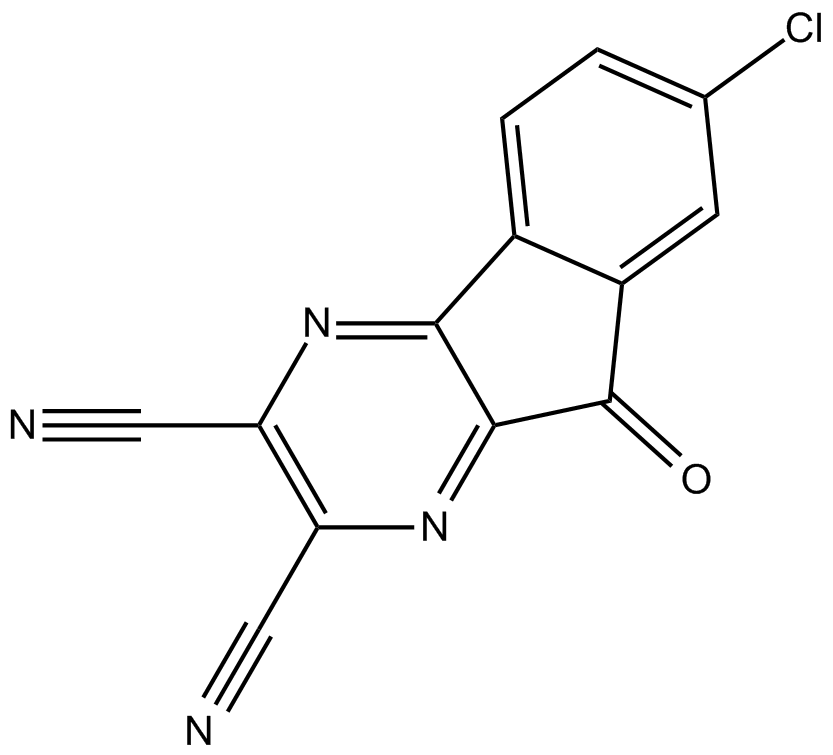 B5550 HBX 41108Summary: ubiquitin-specific protease (USP) 7 inhibitor
B5550 HBX 41108Summary: ubiquitin-specific protease (USP) 7 inhibitor

