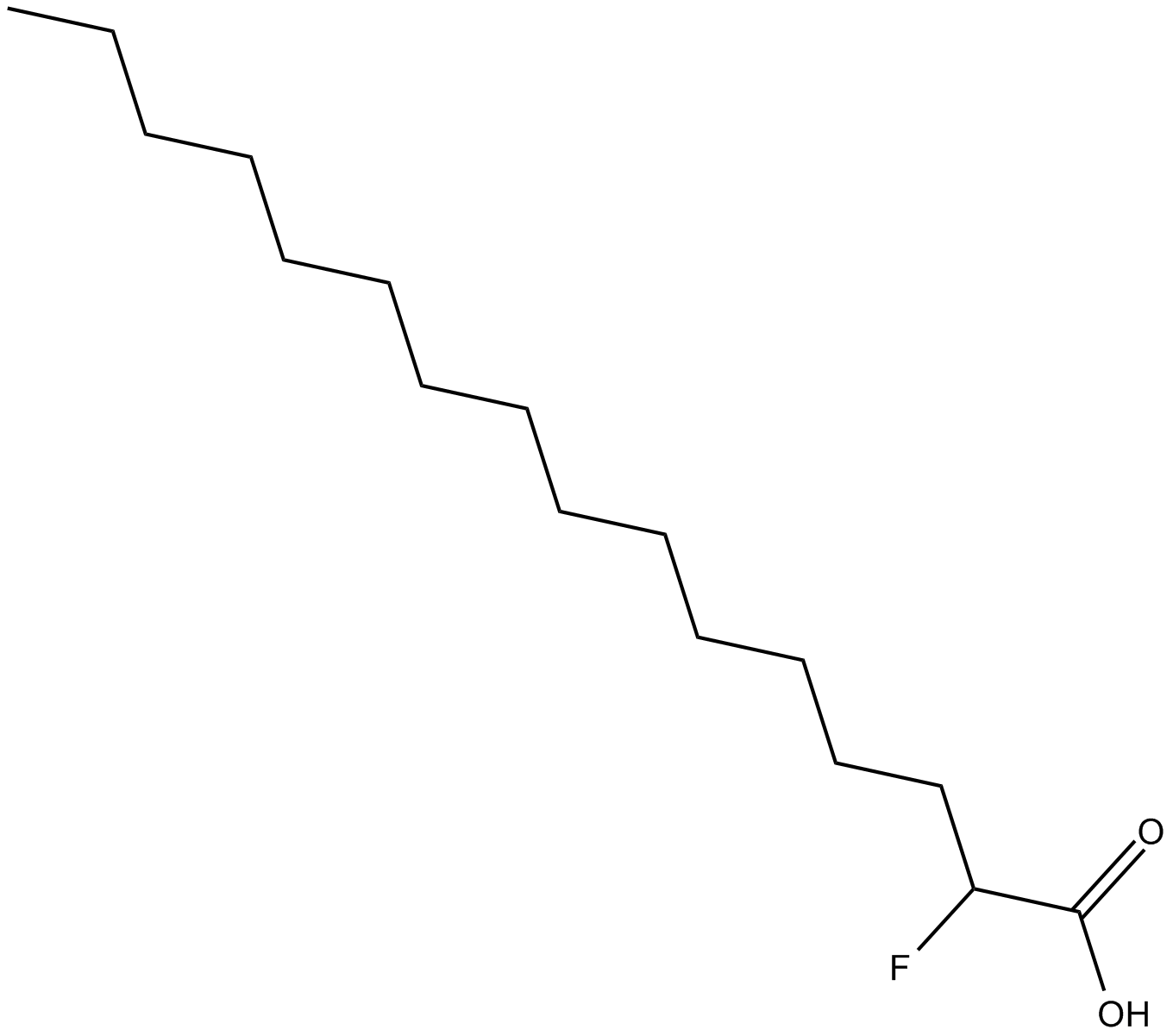
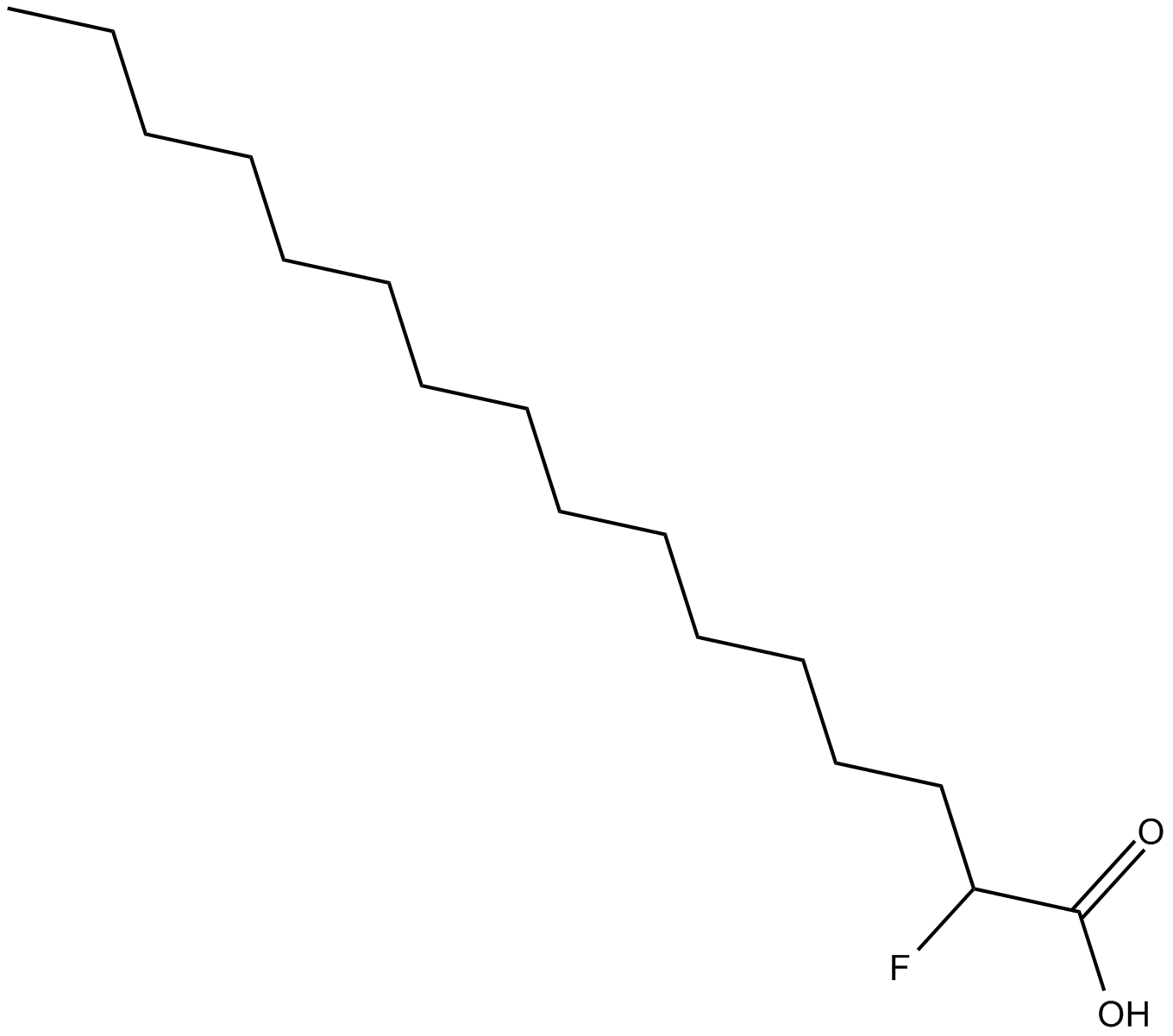
2-fluoro Palmitic Acid
2-fluoropalmitic acid inhibits sphingosine biosynthesis and long-chain acyl-CoA synthetase [1]. The length of the carbon chain of the fatty acid species defines the substrate specificity for the different acyl-CoA synthetases (ACS).
Mammalian long-chain acyl-CoA synthetases (ACSL) activate fatty acids with chain lengths of 12 to 20 carbon atoms. The long-chain acyl-CoA synthetase mRNA is expressed virtually in heart, liver, and epididymal adipose tissues and, to a much lesser extent, in brain, small intestine, and lung [2].
Palmitic acid was a selective cytotoxic substance extracted from the marine algal. At concentrations ranging from 12.5 to 50 μg/ml, palmitic acid showed selective cytotoxicity to human leukemic cells, but no cytotoxicity to normal HDF cells. Palmitic acid (50 μg/ml) induced apoptosis in the human leukemic cell line MOLT-4. Palmitic acid also showed in vivo antitumor activity in mice [3]. 2-fluoropalmitic acid showed an inhibitory effect on sphingosine biosynthesis and long-chain acyl-CoA synthetase with an IC50 value of 0.2 mM [1].
References:
[1] Soltysiak R M, Matsuura F, Bloomer D, et al. d, l-α-fluoropalmitic acid inhibits sphingosine base formation and accumulates in membrane lipids of cultured mammalian cells[J]. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1984, 792(2): 214-226.
[2] Suzuki H, Kawarabayasi Y, Kondo J, et al. Structure and regulation of rat long-chain acyl-CoA synthetase[J]. Journal of Biological Chemistry, 1990, 265(15): 8681-8685.
[3] Harada H, Yamashita U, Kurihara H, et al. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga[J]. Anticancer research, 2001, 22(5): 2587-2590.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 274.4 |
| Cas No. | 16518-94-8 |
| Formula | C16H31FO2 |
| Solubility | Soluble in DMSO |
| Chemical Name | 2-fluorohexadecanoic acid |
| SDF | Download SDF |
| Canonical SMILES | CCCCCCCCCCCCCCC(F)C(=O)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure