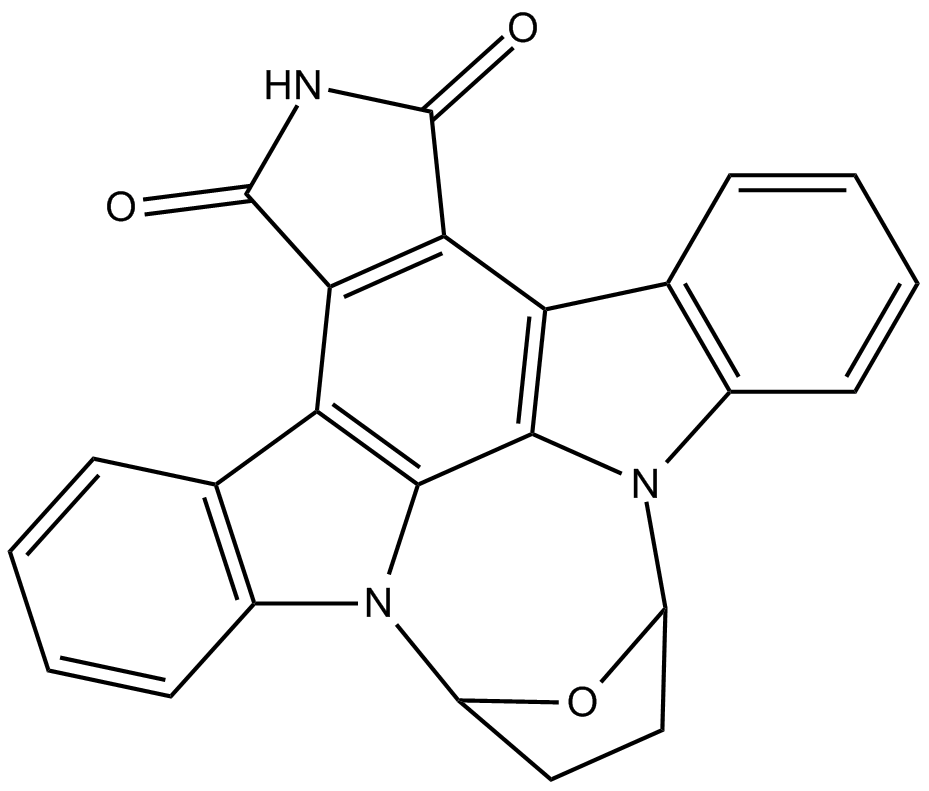
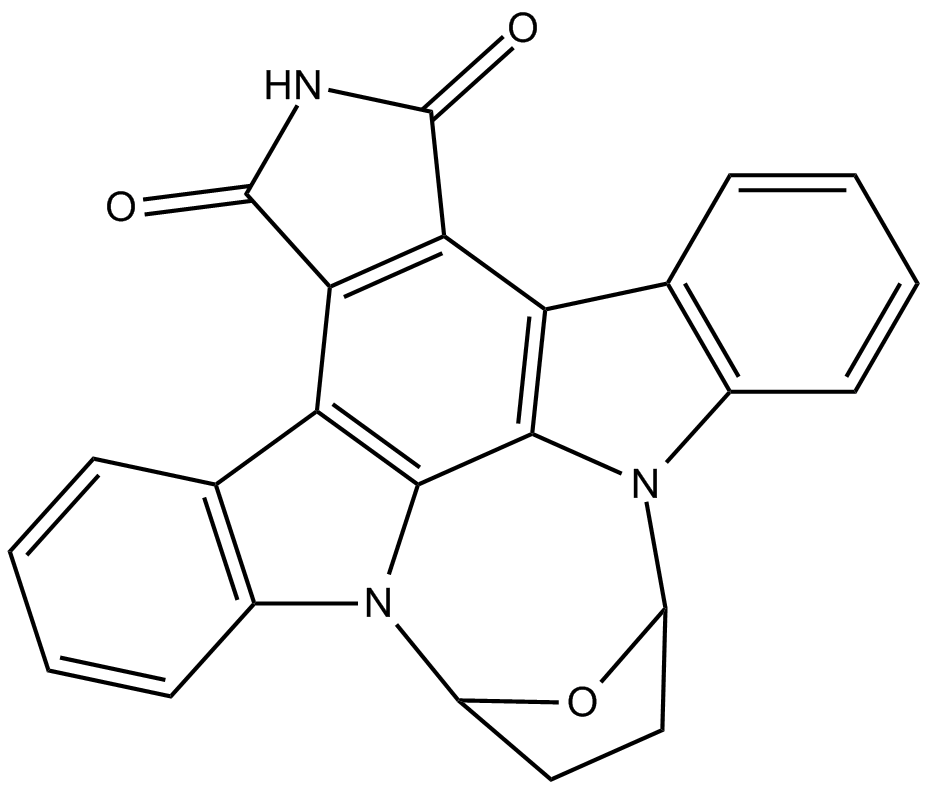
SB 218078
Targets:Chk1, cdc2 and PKC
IC50:15, 250 and 1000 nM for Chk1, cdc2 and PKC respectively
SB 218078 is a potent inhibitor of checkpoint kinase 1, with the IC50 values of 15, 250 and 1000 nM for Chk1, cdc2 and PKC, respectively.
In Vitro: An in vitro kinase assay found that SB 218078 could potently inhibit the phosphorylation of Chk1, with the IC50 of 15 nM. In Hela cells, SB 218078 could cause complete abrogation of G2 cell cycle arrest induced by topotecan, with the minimum concentration of 2.5 μM [1]. Besides, 15 nM SB 218078 could effectively inhibit NKG2D ligand upregulation induced by aphidicolin in fibroblasts [2]. Furthermore, treated GM02188 cells with 2.5 μM SB 218078 could inhibit the formation of HU-induced 53bp1 foci and prevent recovery from replication fork stalling induced by APH [3].
In Vivo: In Myc-induced lymphomas mouse model, treatment with SB 218078 with the dose of 5 mg/kg could promote a strong increase of γ-H2AX and apoptosis throughout the lymphoma, while having no effect on a healthy spleen [4].
Clinical trial: No data available recently
References:
[1] Jackson J R, Gilmartin A G, Imburgia C S, et al. An Indolocarbazole Inhibitor of Human Checkpoint Kinase (Chk1) Abrogates Cell Cycle Arrest Caused by DNA Damage[J]. Cancer Research, 2000, 60(3): 566-572.
[2] Gasser S, Orsulic S, Brown E J, et al. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor[J]. Nature, 2005, 436(7054): 1186-1190.
[3] Alderton G K, Galbiati L, Griffith E, et al. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling[J]. Nature Cell Biology, 2006, 8(7): 725-733.
[4] Murga M, Campaner S, Lopez-Contreras A J, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors[J]. Nature structural & molecular biology, 2011, 18(12): 1331-1335.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 393.4 |
| Cas No. | 135897-06-2 |
| Formula | C24H15N3O3 |
| Solubility | ≤5mg/ml in DMSO;5mg/ml in dimethyl formamide |
| Chemical Name | 9,10,11,12-tetrahydro-9,12-epoxy-1H-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocine-1,3(2H)-dione |
| SDF | Download SDF |
| Canonical SMILES | O=C(NC1=O)C2=C1C3=C4C5=C2C6=C(C=CC=C6)N5C7OC(CC7)N4C8=C3C=CC=C8 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure