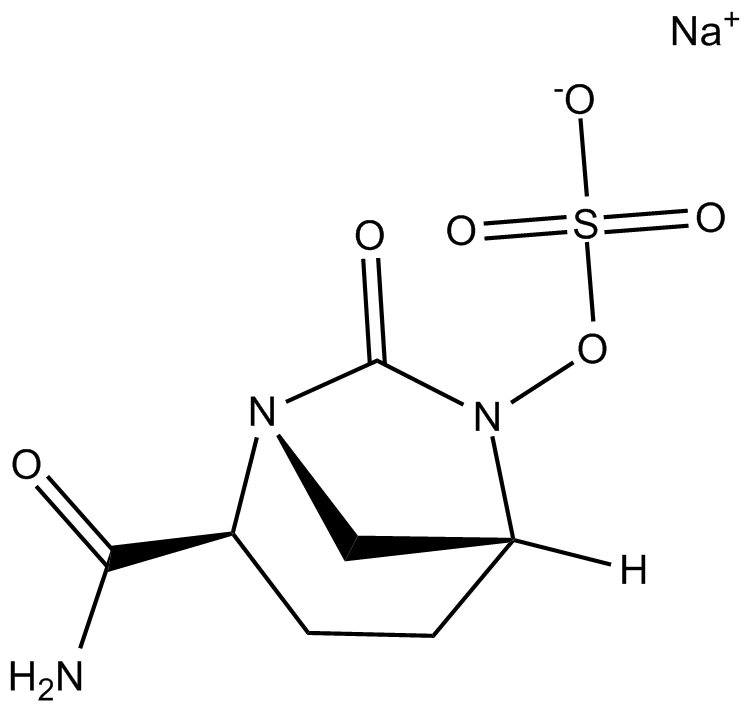
Avibactam sodium
Avibactam sodium is a β-lactamase inhibitor that does not include the β-lactam core but maintains the capacity to covalently acylate its β-lactamase targets. Avibactam sodium inhibits β-lactamases CTX-M-15 and TEM-1, with IC50 values of 5 and 8 nM, respectively. β-Lactamases are enzymes produced by bacteria, with the ability to inactivate β-lactam antibiotics by hydrolyzing the β-lactam ring. β-Lactamases are responsible for resistance to penicillins, monobactams, carbapenems, and extended-spectrum cephalosporins. Avibactam sodium combined with β-lactam antibiotics can be potentially used for the treatment of bacterial infections comprising Gram-negative organisms.
References:
1. Ehmann DE, Jahić H, Ross PL, et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(29): 11663-11668.
2. Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clinical Microbiology Reviews, 2010, 23(1): 160-201.
3. Berkhout J, Melchers MJ, van Mil AC, et al. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrobial Agents and Chemotherapy, 2015, 59(4): 2299-2304.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 287.23 |
| Cas No. | 1192491-61-4 |
| Formula | C7H10N3NaO6S |
| Solubility | ≥28.7 mg/mL in DMSO; insoluble in EtOH; ≥48.2 mg/mL in H2O |
| Chemical Name | sodium (1R,2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl sulfate |
| SDF | Download SDF |
| Canonical SMILES | O=S(ON1[C@@]2(CC[C@H]([N@](C1=O)C2)C(N)=O)[H])([O-])=O.[Na+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Animal experiment:[3] | |
|
Animal models |
Mice infected with about 106 CFU of Pseudomonas aeruginosa intramuscularly into the thigh or intranasally |
|
Dosage form |
1 ~ 128 mg/kg Subcutaneous administration |
|
Applications |
No pharmacokinetic interaction between ceftazidime and avibactam was observed. Ceftazidime and avibactam showed linear plasma pharmacokinetics that were independent of the dose combinations used or the infection site in mice. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Ehmann DE, Jahić H, Ross PL, et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(29): 11663-11668. 2. Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clinical Microbiology Reviews, 2010, 23(1): 160-201. 3. Berkhout J, Melchers MJ, van Mil AC, et al. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrobial Agents and Chemotherapy, 2015, 59(4): 2299-2304. |
|
Quality Control & MSDS
- View current batch:
Chemical structure