Pertussis Toxin
Protein-based AB5-type exotoxin Pertussis toxin (PT) is produced by the bacterium Bordetella pertussis. PT can cause whooping cough.
In vitro: PT has the effect on innate immune response. The effects of PT on human monocyte-derived DC (MDDC), including maturation, are regulated by cAMP [1].
In vivo: Pertussis toxin did not affect the relaxation and contraction properties of isolated NIH or BALB/c mouse trachea, but significantly declined KCl or noradrenaline-induced maximal contraction and decreased sensitivity to noradrenaline in isolated male Wistar rat small mesenteric resistance arteries [2].
Clinical trial: Pertussis toxin was used as vaccine, acellular pertussis booster immunizations, to young and older persons in order to reduce the community transmission and to enhance the protection. Older persons with infections are mainly asymptomatic. Acellular pertussis boosters provide protection against symptomatic pertussis and give protection against mild and asymptomatic infections. The use of boosters may reduce transmission rate, especially in infant population [3].
References:
[1] Bagley KC, Abdelwahab SF, Tuskan RG, Fouts TR, Lewis GK. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J Leukoc Biol. 2002 Nov;72(5):962-9.
[2] van Meijeren CE, Vleeming W, van de Kuil T, Manni J, Kegler D, Hendriksen CF, de Wildt DJ. In vivo pertussis toxin treatment reduces contraction of rat resistance arteries but not that of mouse trachea. Eur J Pharmacol. 2004 Mar 19; 488 (1-3): 127-35.
[3] Ward JI, Cherry JD, Chang SJ, Partridge S, Keitel W, Edwards K, Lee M, Treanor J, Greenberg DP, Barenkamp S, Bernstein DI, Edelman R; APERT Study Group. Bordetella Pertussis infections in vaccinated and unvaccinated adolescents and adults, as assessed in a national prospective randomized Acellular Pertussis Vaccine Trial (APERT). Clin Infect Dis. 2006 Jul 15; 43 (2): 151-7. Epub 2006 Jun 5.
- 1. Liming Yu, Yujun Wen, et al. "Autoimmune receptor encephalitis in ApoE‑/‑mice induced by active immunization with NMDA1." Mol Med Rep. 2023 Dec;28(6):233. PMID: 37921064
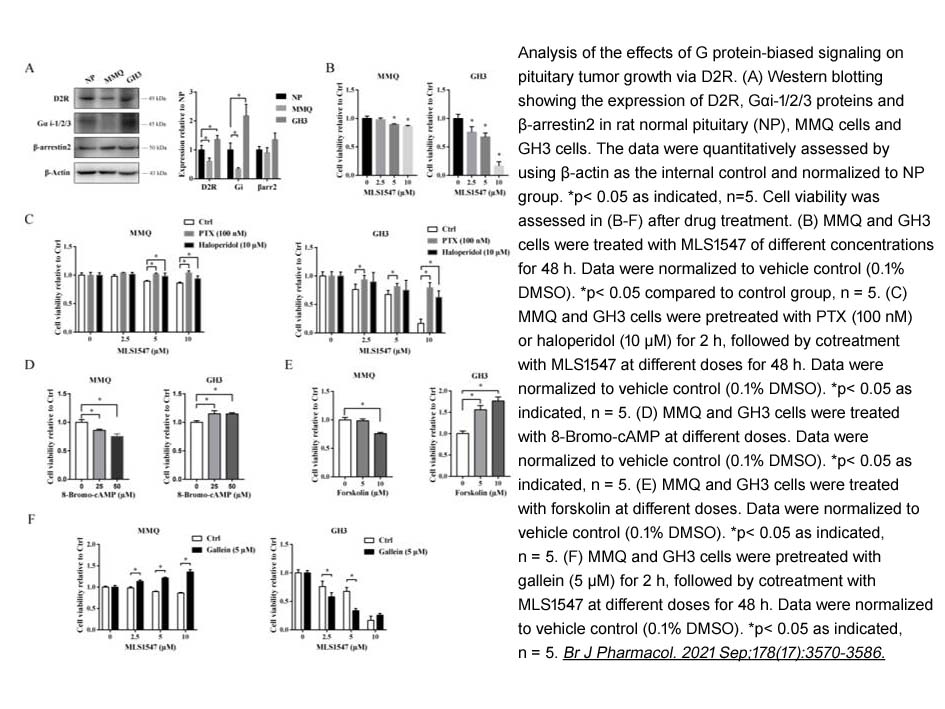
- 2. Zhoubin Tan, Zhuowei Lei, et al. "Exploiting D2receptor β‐arrestin2‐biased signalling to suppress tumour growth of pituitary adenomas." Br J Pharmacol. 2021 Sep;178(17):3570-3586. PMID:33904172
- 3. Zhou H, Peng X, et al. "Identification of novel phytocannabinoids from Ganoderma by label-free dynamic mass redistribution assay." J Ethnopharmacol. 2019 Sep 5:112218. PMID:31494202
| Physical Appearance | Each vial, when reconstituted to 500 μL with water, contains 50 μg of pertussis toxin in 0.01 M sodium phosphate, 0.05 M sodium chloride, pH 7.0 |
| Storage | Desiccate at 4°C |
| Cas No. | 70323-44-3 |
| Solubility | Soluble in H2O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Related Biological Data