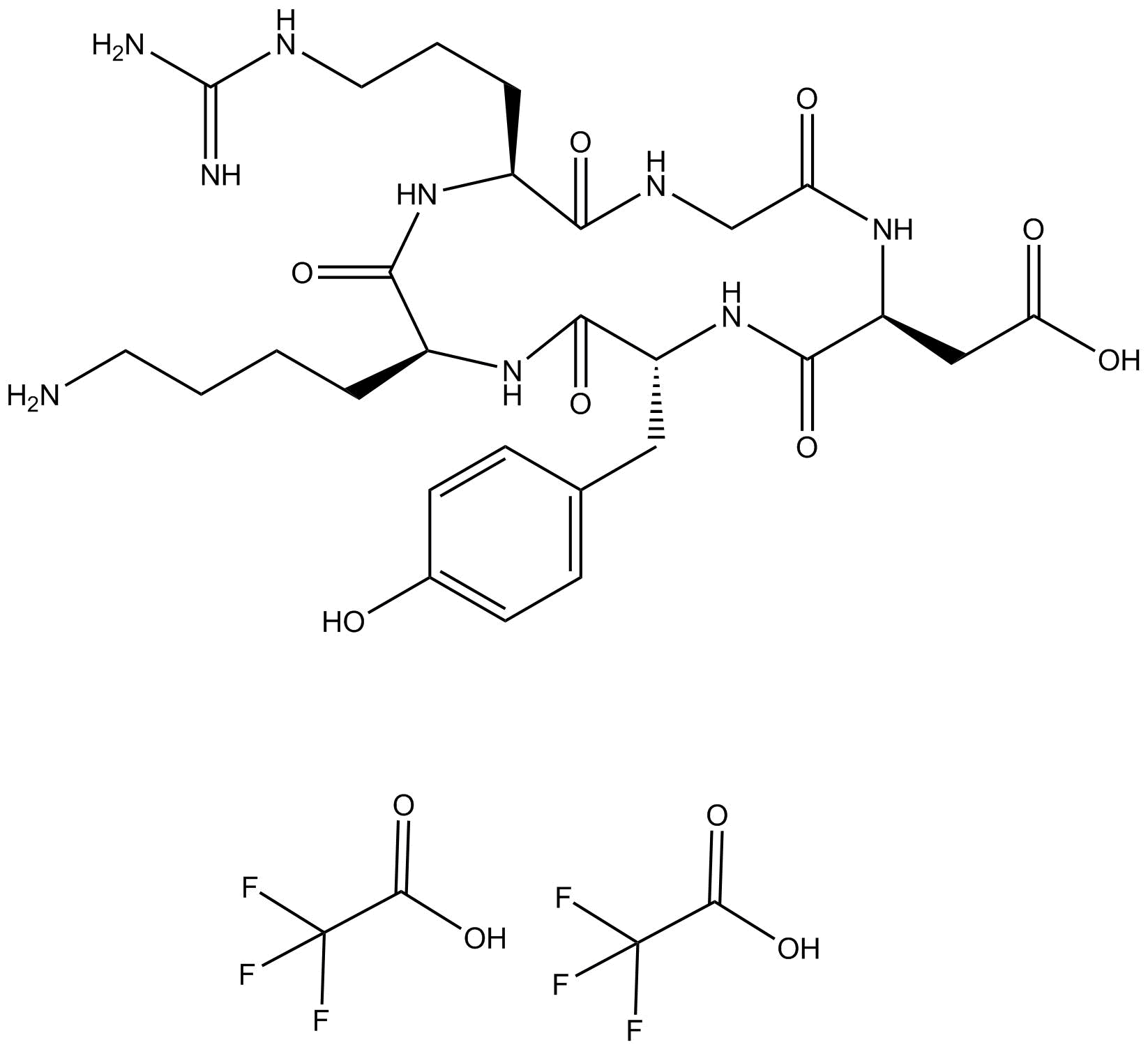
Cyclo(RGDyK)
Target: αVβ3 integrin
IC50: 20 nM
Cyclo(RGDyK) is a selective and potent αVβ3 integrin inhibitor with IC50 value of 20 nM [1]. The αVβ3 integrin, expressed on various malignant human tumors and on endothelial cells, plays an important role in tumor-induced angiogenesis and tumor metastasis. Therefore, αVβ3 integrin is a potential therapeutic target for tumor disease. Inhibition of αVβ3 integrin by αVβ3 antagonists not only blocked blood vessel formation but further led to tumor regression [1].
In vitro: Cyclo(RGDyK) conjugation facilitated intracellular drug delivery of polymeric micelles to neovasculature (HUVECs) and tumor cells in which integrin is overexpressed [2]. In addition, Cyclo(RGDyK) showed high affinity and selectivity for αVβ3 integrin over αVβ5 and αIIbβ3 [1].
In vivo: Cyclo(RGDyK) (1 nM, i.v. injection) blocked the increase of αVβ3 integrin expression in the intima of the left stenotic carotid artery of apoE-/- mice [2].
References:
1. Haubner R, Wester HJ, Burkhart F, Senekowitsch-Schmidtke R, Weber W, Goodman SL, et al. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42(2):326-36.
2. Yin J, Li Z, Yang T, Wang J, Zhang X, Zhang Q. Cyclic RGDyK conjugation facilitates intracellular drug delivery of polymeric micelles to integrin-overexpressing tumor cells and neovasculature. J Drug Target. 2011;19(1):25-36.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 847.72 |
| Cas No. | 250612-42-1 |
| Formula | C31H43F6N9O12 |
| Solubility | insoluble in EtOH; ≥50.2 mg/mL in H2O with ultrasonic; ≥6.347 mg/mL in DMSO with gentle warming and ultrasonic |
| Chemical Name | 2,2,2-trifluoroacetic acid compound with 2-((1Z,2S,3Z,5R,6Z,8S,9Z,11S,12Z)-8-(4-aminobutyl)-11-(3-guanidinopropyl)-3,6,9,12,15-pentahydroxy-5-(4-hydroxybenzyl)-1,4,7,10,13-pentaazacyclopentadeca-3,6,9,12,15-pentaen-2-yl)acetic acid (2:1) |
| SDF | Download SDF |
| Canonical SMILES | NCCCC[C@@]1([H])/C(O)=N/[C@@](/C(O)=N/C/C(O)=N/[C@@](/C(O)=N/[C@](/C(O)=N/1)([H])CC2=CC=C(O)C=C2)([H])CC(O)=O)([H])CCCNC(N)=N.FC(F)(F)C(O)=O.FC(F)(F)C(O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure