Erteberel (LY500307)
Erteberel (LY500307) is a potent and selective ERβ (estrogen receptor beta) agonist (EC50 = 0.66 nM). [1]
Estrogen receptors are nuclear hormone receptors that act as a ligand-activated transcription factor. It regulates gene expression, cell proliferation and differentiation in target tissues and involved in breast cancer, endometrial cancer and osteoporosis etc. [1]
In transcription assay of cotransfected human prostate cancer cell line, Erteberel showed potency (EC50 = 0.66 nM), selectivity (32-fold) and full agonist function (>90% relative efficacy) to ERβ than to ERα. [1]
In mouse model, prostate wet weight of CD-1 mice were measured following oral daily doses of Erteberel for 7 days. Results showed reduction on prostate weight in a dose-dependent manner (0.01 mg/kg – 0.05 mg/kg), and no effect on testes/seminal vesicle weight and androgens testosterone/dihydrotestosterone circulating level. [1]
Reference:
[1] Norman BH, Dodge JA, Richardson TI, Borromeo PS, Lugar CW, Jones SA, Chen K, Wang Y, Durst GL, Barr RJ, Montrose-Rafizadeh C, Osborne HE, Amos RM, Guo S, Boodhoo A, Krishnan V. Benzopyrans are selective estrogen receptor beta agonists with novel activity in models of benign prostatic hyperplasia. J Med Chem. 2006 Oct 19;49(21):6155-7.
- 1. Dongfang Wang, Muya Tang, et al. "Activation of ERβ hijacks the splicing machinery to trigger R-loop formation in triple-negative breast cancer." Proc Natl Acad Sci U S A. 2024 Mar 26;121(13):e2306814121 PMID: 38513102
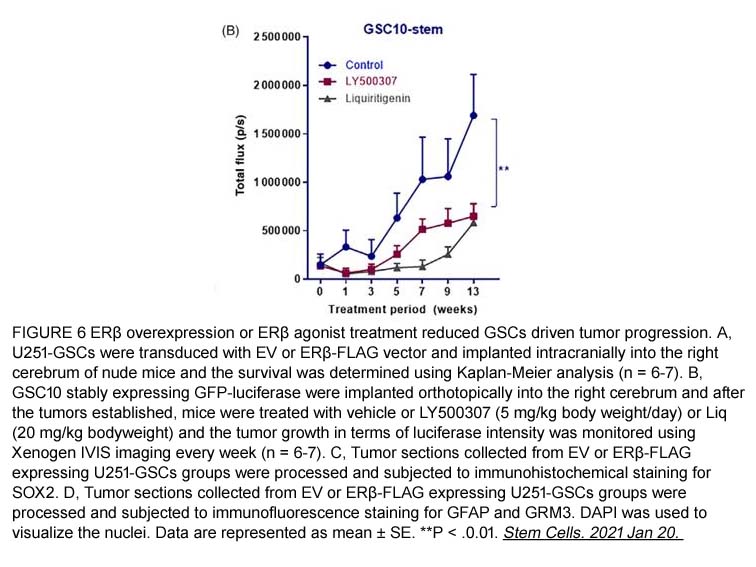
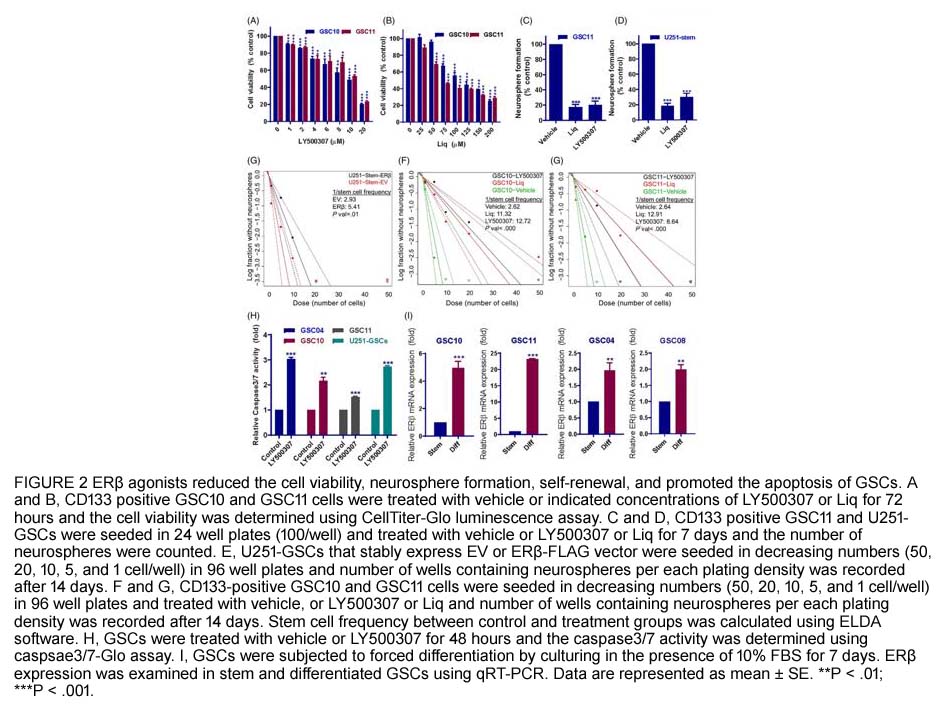
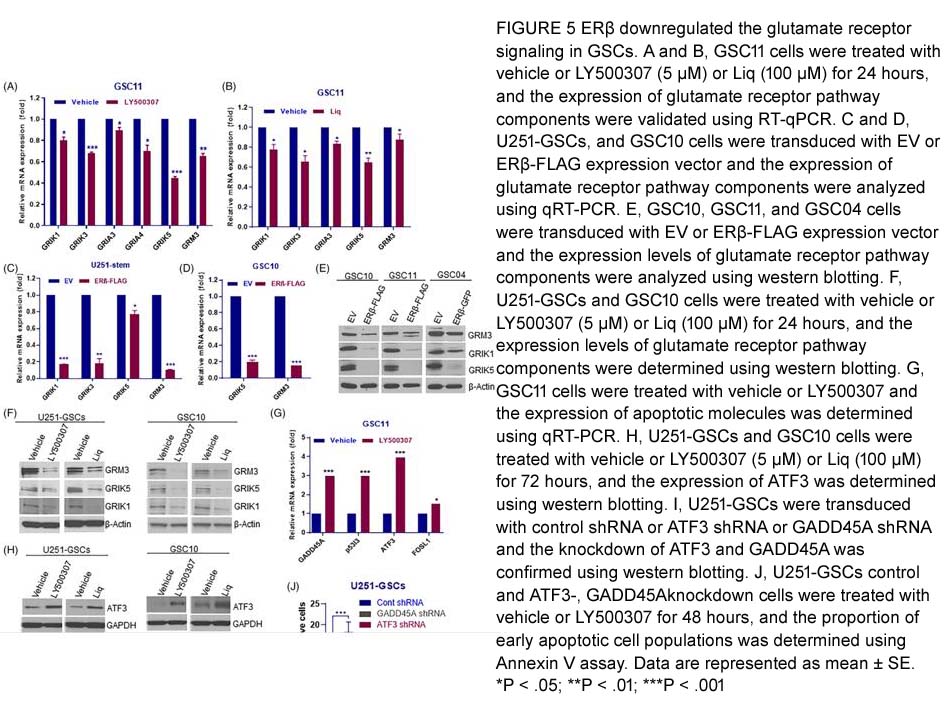
- 2. Gangadhara R. Sareddy, Uday P. Pratap, et al. "Activation of estrogen receptor beta signaling reduces stemness of glioma stem cells." Stem Cells. 2021 May;39(5):536-550. PMID:33470499
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 282.33 |
| Cas No. | 533884-09-2 |
| Formula | C18H18O3 |
| Solubility | insoluble in H2O; ≥14.1 mg/mL in DMSO; ≥48.3 mg/mL in EtOH |
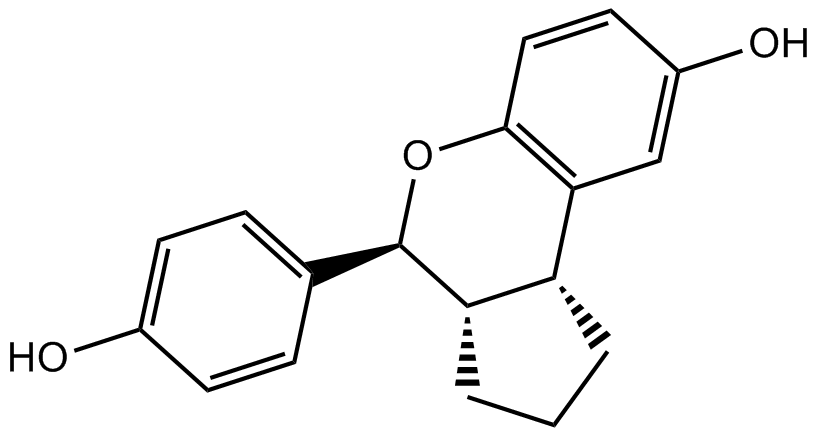
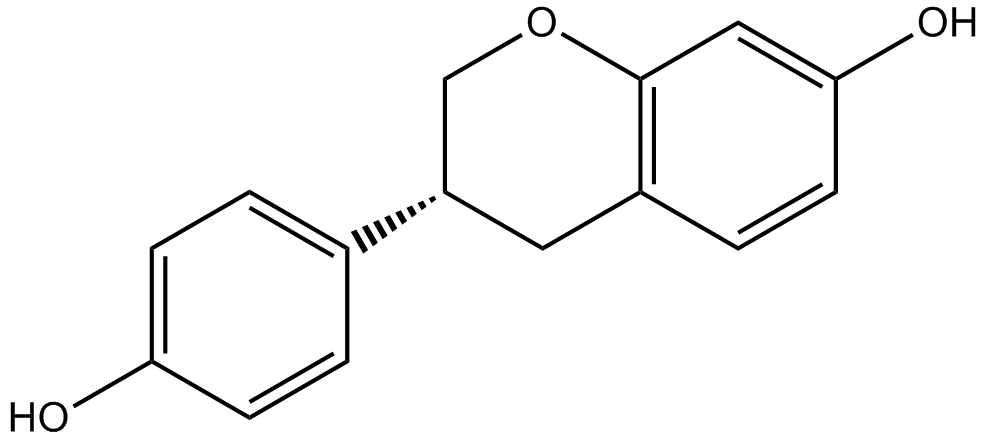
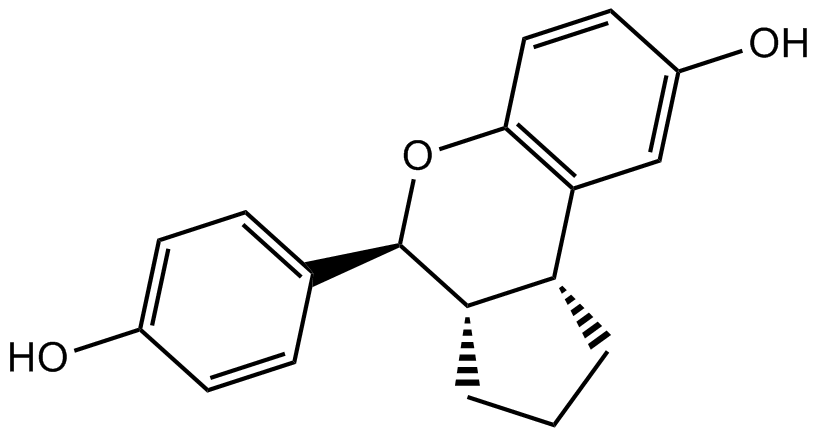
| Chemical Name | (3aS,4R,9bR)-4-(4-hydroxyphenyl)-1,2,3,3a,4,9b-hexahydrocyclopenta[c]chromen-8-ol |
| SDF | Download SDF |
| Canonical SMILES | C1CC2C(C1)C3=C(C=CC(=C3)O)OC2C4=CC=C(C=C4)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
ER Binding Assays |
Competition binding assay is run in a buffer containing 50mM Hepes, pH 7.5, 1.5mM EDTA, 150mM NaCl, 10% glycerol, 1mg/ml ovalbumin and 5mM DTT, using 0.025 μCi per well 3HEstradiol, 10 ng/well ERalpha or ERbeta receptor. Competing compounds are added at 10 different concentrations. Non-specific binding is determined in the presence of 1μM of 17-β Estradiol. The binding reaction (140 μl) is incubated for 4 hours at room temperature, then 70 μl of cold DCC buffer is added to each reaction (DCC buffer contains per 50 ml of assay buffer, 0.75g of charcoal and 0.25g of dextran). Plates are mixed 8 minutes on an orbital shaker at 4°C. Plates are then centrifuged at 3,000 rpm at 4°C for 10 minutes. An aliquot of 120μl of the mix is transferred to another 96-well, white flat bottom plate and 175μl of Wallac Optiphase “Hisafe 3” scintillation fluid is added to each well. Plates are sealed and shaken vigorously on an orbital shaker. After an incubation of ~5 hrs, read plates in a Wallac Microbeta counter. The data is used to calculate an IC50 and % Inhibition at 10μM. The Kd for 3H-Estradiol is determined by saturation binding to ER alpha and ER beta receptors. The IC50 values for compounds are converted to Ki using Cheng-Prusoff equation and the Kd determined by saturation binding assay. |
| Cell experiment: | |
|
Cell lines |
Human prostate cancer cell line (PC-3 cells) |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
N/A |
|
Applications |
Erteberel showed potent and selective binding affinity for ERβ with EC50 value of 0.66 nM [1]. |
| Animal experiment: | |
|
Animal models |
Male and female rat fertility and rat and rabbit embryo-fetal development model |
|
Dosage form |
0.03 to 10 mg/kg/day for rats, or 1 to 25 mg/kg/day for rabbits, oral gavage, for 2 or 10 weeks |
|
Applications |
There were no-observed adverse effect levels following LY500307 administration of 1 mg/kg/day for male rat fertility, 0.3 mg/kg/day for female rat fertility and embryo-fetal development, and 25 mg/kg/day for rabbit embryo-fetal development [2]. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: 1. Norman, B. H., Dodge, J. A., Richardson, T. I., Borromeo, P. S., Lugar, C. W., Jones, S. A., Chen, K., Wang, Y., Durst, G. L., Barr, R. J., Montrose-Rafizadeh, C., Osborne, H. E., Amos, R. M., Guo, S., Boodhoo, A. and Krishnan, V. (2006) Benzopyrans are selective estrogen receptor beta agonists with novel activity in models of benign prostatic hyperplasia. J Med Chem. 49, 6155-6157 2. Hilbish, K. G., Breslin, W. J., Johnson, J. T. and Sloter, E. D. (2013) Fertility and developmental toxicity assessment in rats and rabbits with LY500307, a selective estrogen receptor beta (ERbeta) agonist. Birth Defects Res B Dev Reprod Toxicol. 98, 400-415 |
|
Quality Control & MSDS
- View current batch:
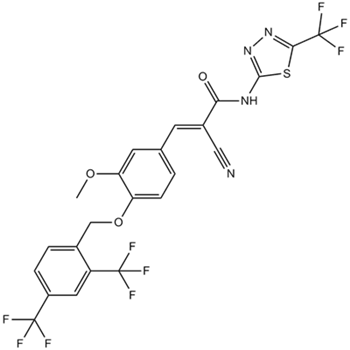
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data