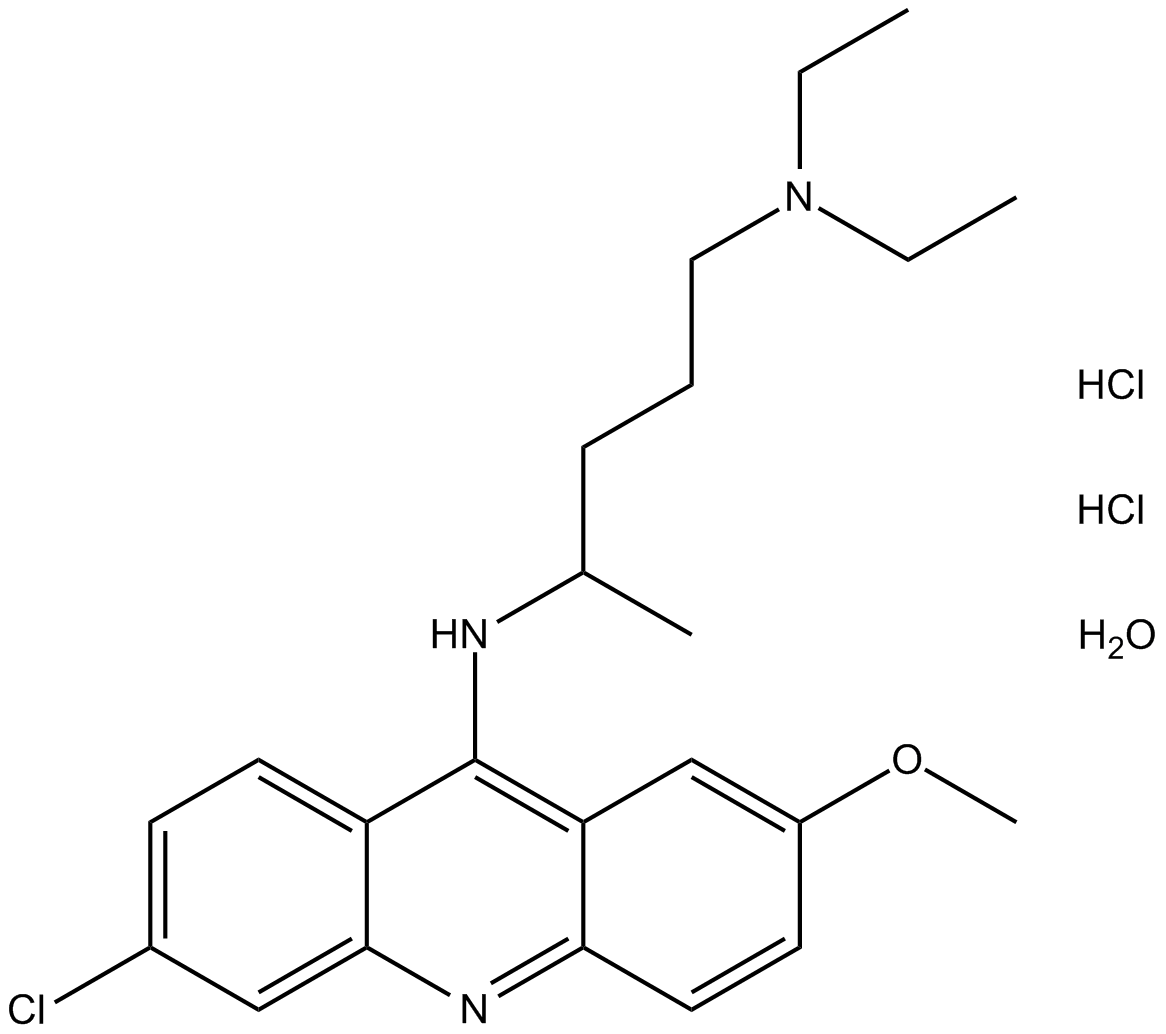
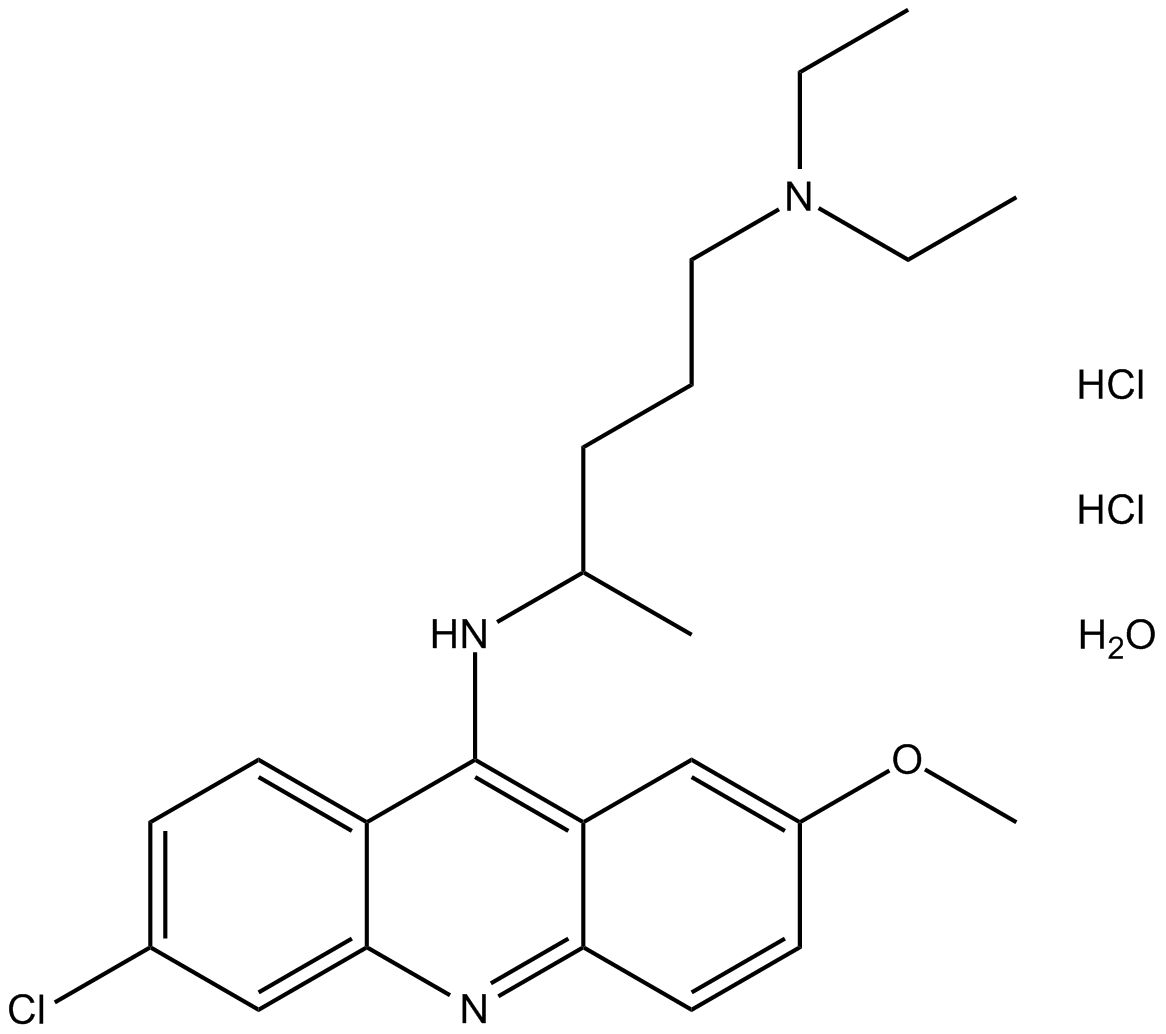
Quinacrine (hydrochloride hydrate)
IC50: 3.3 μM
Ki = 6.7 μM
Quinacrine, also commonly known as atebrine, is a compound which is commonly used as an anti-protozoal agent. It inhibits voltage-dependent sodium channels with an IC50 value of 3.3 μM and suppresses aldehyde oxidase with an IC50 value of 3.3 μM. Quinacrine prevents misfolding of prion protein with an EC50 value of 0.3 μM. As an effective riboflavin antagonist, quinacrine associates with the riboflavin-binding protein with a Ki value of 6.7 μM. Voltage-dependent sodium channels play a vital role in action potential initiation and propagation in excitable cells, including muscle, nerve, and neuroendocrine cell types.
In vitro: Quinacrine, in a dose-dependent manner, effectively reversed the resistance in the multi-drug resistance (MDR) K562 cells. Quinacrine displayed strong toxicity to the MDR K562 cells at a concentration of 10.0 μM. Compared to the control, quinacrine significantly increased the activity of caspase-9 and -3 activities in the MDR K562 and K562 cells in a dose-dependent fashion [1].
In vivo: Female BALB/c nude mice, bearing MDR K562 cell xenografts, were injected with quinacrine at a dose of 10 mg/kg via tail vein for 13 days. Compared to the control group, quinacrine inhibited the tumor growth obviously in the treated groups. Furthermore, quinacrine enhanced the anti-tumor effects of vincristine [1].
Reference:
Liang, G. , Lu, W., Wu, J., Zhao, J., Hong, H., & Long, C. et al. Enhanced therapeutic effects on the multi-drug resistant human leukemia cells in vitro and xenograft in mice using the stealthy liposomal vincristine plus quinacrine. Fundamental & Clinical Pharmacology.2008; 22(4): 429-437.
| Storage | Store at -20°C |
| M.Wt | 472.9 |
| Formula | C23H30ClN3O·2HCl [XH2O] |
| Synonyms | Atebrine,Mepacrine |
| Solubility | insoluble in DMSO; ≥18.65 mg/mL in H2O; ≥2.62 mg/mL in EtOH with ultrasonic |
| Chemical Name | N4-(6-chloro-2-methoxyacridin-9-yl)-N1,N1-diethylpentane-1,4-diamine, dihydrochloride hydrate |
| SDF | Download SDF |
| Canonical SMILES | ClC1=CC=C2C(NC(C)CCCN(CC)CC)=C3C(C=CC(OC)=C3)=NC2=C1.Cl.Cl.O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure