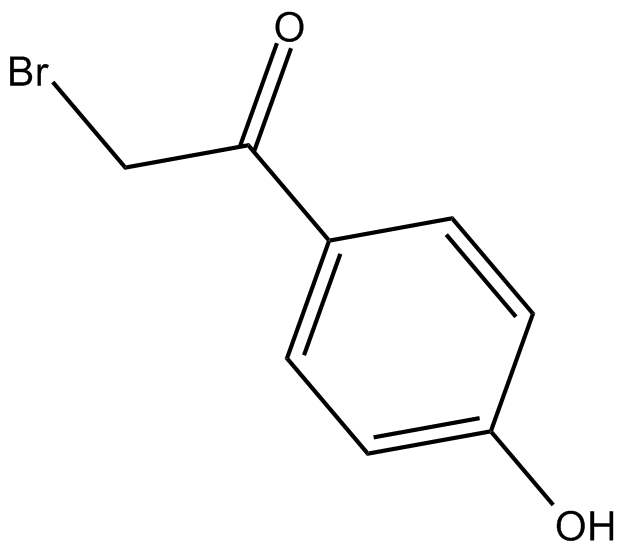
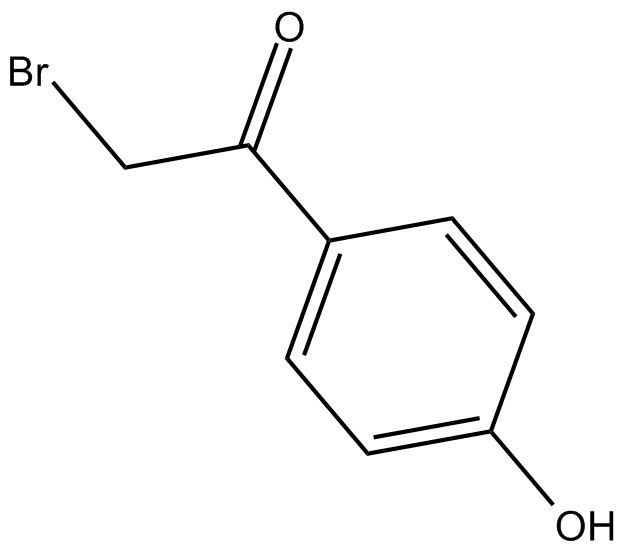
PTP Inhibitor I
KI: 43 and 42 μM for SHP-1 and PTP1B, respectively.
PTP Inhibitor I is a tyrosine phosphatase (PTP) inhibitor.
Protein tyrosine phosphatases (PTPs) are considered to be involved in the etiology of diabetes mellitus, neural diseases such as Alzheimer’s and Parkinson’s disease, regulation of allergy and inflammation. PTPs are also considered to be responsible for the pathogens’ virulence.
In vitro: In previous study, the corresponding values of PTP Inhibitor I against PTP1B were determined to be KI of 42 μM, kinact of 0.57 min-1, and kinact/KI of 1.4*104 M-1 min-1, respectively. This study also showed that α-bromoacetophenone such as PTP Inhibitor I could provide an effective, neutral pY mimetic inhibitor of PTPs. While perturbation of the electronic properties of the phenyl ring did not significantly improve its potency against PTPs, attachment of a proper peptidyl moiety to the para position could improve both the potency and the selectivity substantially. In addition, since the covalent PTP inhibitor complex could be cleaved to regenerate the PTP activity photolytically, PTP Inhibitor I might provide a novel class of photolytic switch for controlling cellular signaling processes [1].
In vivo: Currently, there is no animal in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] Arabaci G, Yi T, Fu H, Porter ME, Beebe KD, Pei D. alpha-bromoacetophenone derivatives as neutral protein tyrosine phosphatase inhibitors: structure-Activity relationship. Bioorg Med Chem Lett. 2002 Nov 4;12(21):3047-50.
| Storage | Store at RT |
| M.Wt | 215.0 |
| Cas No. | 2491-38-5 |
| Formula | C8H7BrO2 |
| Synonyms | α-Bromo-4-hydroxyacetophenone|2-Bromo-4'-hydroxyacetophenone|4-Hydroxyphenacyl bromide|Protein Tyrosine Phosphatase Inhibitor I|SHP-1 Inhibitor II |
| Solubility | insoluble in H2O; ≥11.5 mg/mL in DMSO; ≥33.35 mg/mL in EtOH |
| Chemical Name | 2-bromo-1-(4-hydroxyphenyl)-ethanone |
| SDF | Download SDF |
| Canonical SMILES | Oc(cc1)ccc1C(CBr)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure