Meleagrin
MIC: 32-64 μg/ml for S. aureus, E. coli, and S. pneumoniae
Meleagrin is an antibiotic.
Meleagrin is an antibiotic derived from a deep ocean, penicillin-producing P. chrysogenum.
In vitro: It was found that consistent with their selective inhibition of Staphylococcus aureus FabI, meleagrin and its more active derivatives could directly bind to S. aureus FabI that was measured in a fluorescence quenching assay, inhibit intracellular fatty acid biosynthesis and growth of S. aureus, and increase the minimum inhibitory concentration (MIC) for fabI-overexpressing S. aureus. The compounds that were not effective against the FabK isoform, however, were able to inhibit the growth of Streptococcus pneumoniae containing only the FabK isoform. In addition, no resistant mutant to these compounds was obtained. Notely, fabK-overexpressing Escherichia coli was found to be not resistant to these compounds, but was resistant to triclosan [1]. Another study found that meleagrin was able to inhibit the growth of the human breast cancer cell lines, while similar treatment doses had no effect on the growth and viability of the non-tumorigenic human mammary epithelial cells MCF10A. Meleagrin also displayed good ATP competitive c-Met inhibitory activity in Z-Lyte assay [2].
In vivo: Up to now, there is no animal in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Zheng, C. J.,Sohn, M.J.,Lee, S., et al. Meleagrin, a new FabI inhibitor from Penicillium chryosogenum with at least one additional mode of action. PLoS One 8(11), (2013).
[2] Mady MS et al. The indole alkaloid meleagrin, from the olive tree endophytic fungus Penicillium chrysogenum, as a novel lead for the control of c-Met-dependent breast cancer proliferation, migration and invasion. Bioorg Med Chem. 2016 Jan 15;24(2):113-22.
| Physical Appearance | A light tan to tan solid |
| Storage | Store at -20°C |
| M.Wt | 433.5 |
| Cas No. | 71751-77-4 |
| Formula | C23H23N5O4 |
| Synonyms | 6-O-Methyloxaline |
| Solubility | Soluble in ethanol;Soluble in methanol;Soluble in DMSO;Soluble in dimethyl formamide |
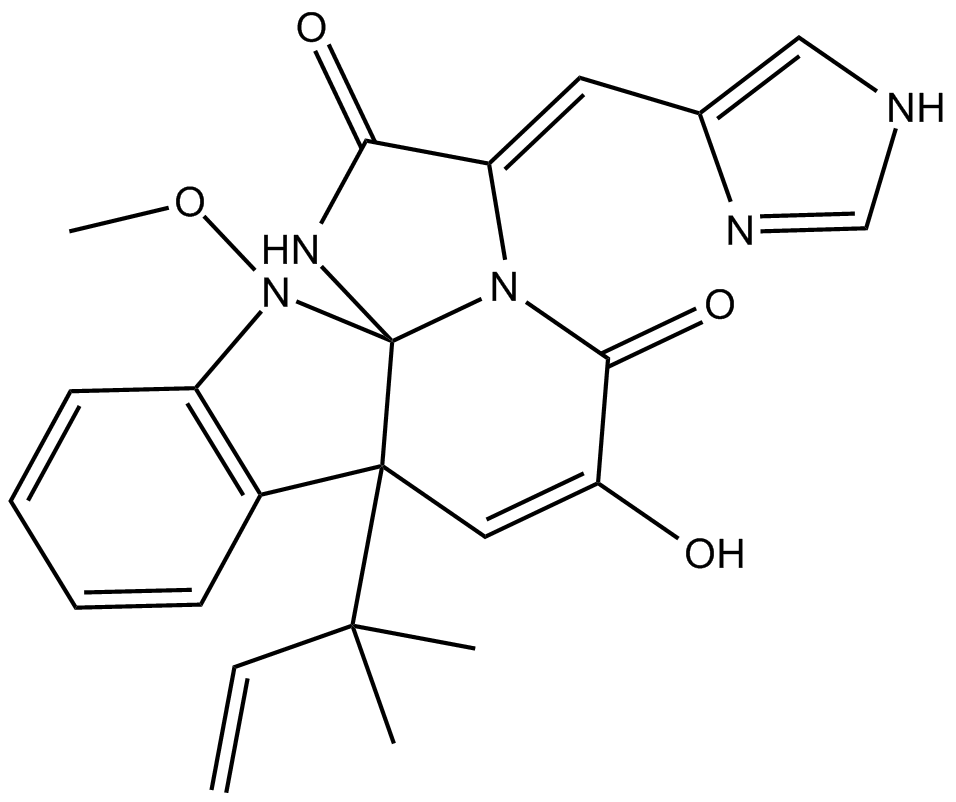
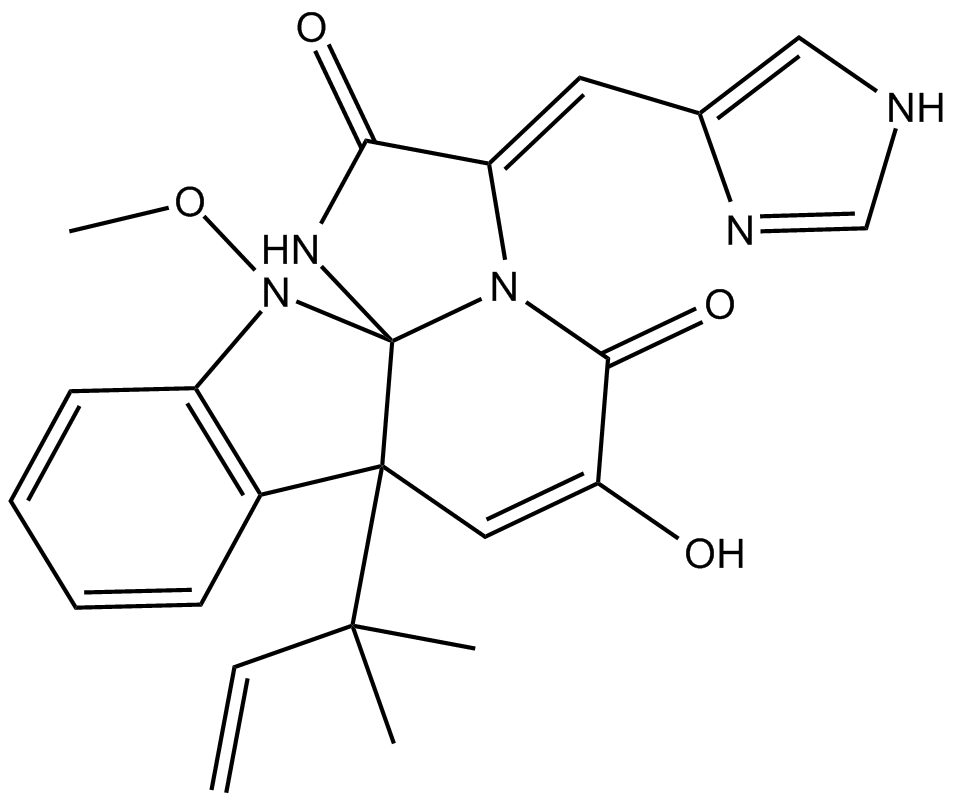
| Chemical Name | (3E,7aR,12aS)-7a-(1,1-dimethyl-2-propen-1-yl)-7a,12-dihydro-6-hydroxy-3-(1H-imidazol-5-ylmethylene)-12-methoxy-1H,5H-imidazo[1',2':1,2]pyrido[2,3-b]indole-2,5(3H)-dione |
| SDF | Download SDF |
| Canonical SMILES | CC(C)([C@]([C@]1(NC(/C2=C/c3c[nH]cn3)=O)N/2C2=O)(C=C2O)c(cccc2)c2N1OC)C=C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Human breast cancer cell lines |
|
Preparation method |
The solubility of this compound in DMSO is soluble. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0~50 μM |
|
Applications |
Meleagrin inhibited the growth of human breast cancer cells MDA-MB-231, MDA-468, BT-474, SK BR-3, MCF7 and MCF7-dox. It was also found that similar therapeutic had no effect on the growth and viability of non-tumorigenic human mammary epithelial cells MCF10A. Meleagrin also showed excellent ATP-competitive c-Met inhibitory activity in Z-Lyte analysis, which had been further confirmed by molecular docking studies and Western blot analysis. |
|
References: [1]. Mady MS, Mohyeldin MM, Ebrahim HY, Elsayed HE, Houssen WE, Haggag EG, Soliman RF, El Sayed KA. The indole alkaloid meleagrin, from the olive tree endophytic fungus Penicillium chrysogenum, as a novel lead for the control of c-Met-dependent breast cancer proliferation, migration and invasion. Bioorg Med Chem. 2016 Jan 15;24(2):113-22. doi: 10.1016/j.bmc.2015.11.038. Epub 2015 Nov 28. PMID: 26692349; PMCID: PMC4703546. |
|
Quality Control & MSDS
- View current batch:
-
Purity = 98.08%
- COA (Certificate Of Analysis)
- HPLC
- MSDS (Material Safety Data Sheet)
Chemical structure