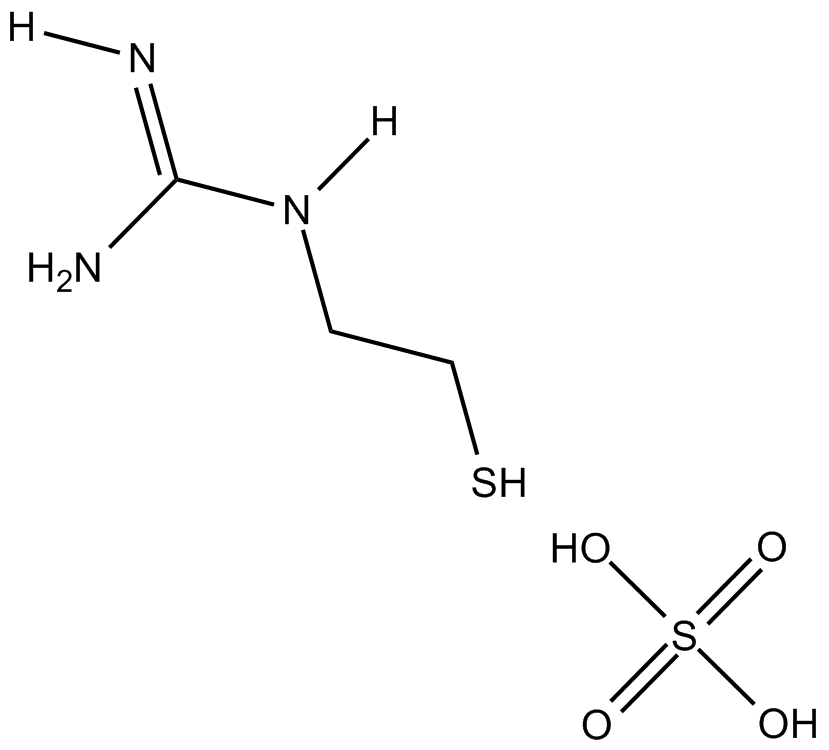
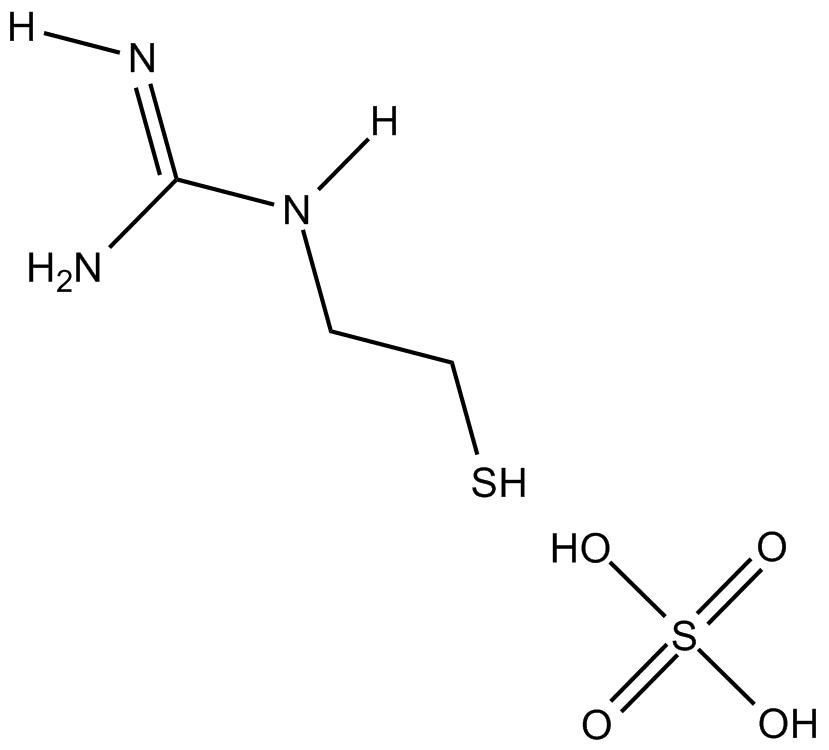
MEG (sulfate)
MEG is a selective inhibitor of the inducible NO synthase (iNOS) [1].
NO synthase catalyses the oxidation of the amino acid L-arginine to citrulline and NO. Low concentrations of NO stimulate guanylate cyclase activity and trigger the formation of cyclic GMP (cGMP), an important messenger mediating physiological functions of NO such as vascular homeostasis. At higher concentrations, NO produced by iNOS interact with thiol groups or transition-metalcontaining proteins and can alter protein function or initiate gene expression to protect cells [2].
In vitro: In tissue homogenates, the EC50 values of MEG for inhibition of iNOS (LPS-treated rat lung), eNOS (bovine endothelial), and nNOS (rat brain) were 11.5, 110, and 60 μM, respectively [1]. MEG reduced nitrite/nitrate concentrations in the exudate and the activity of the inducible isoform of NO synthase in the lung ex vivo. In the inflamed tissues, MEG reduced the appearance of nitrotyrosine immunoreactivity [3].
In vivo: Mercaptoethylguanidine (MEG) slightly decreased the mean arterial blood pressure (MAP) in control rats. In endotoxin-treated rats, MEG increased MAP and restored 80% of the endotoxin-induced fall in MAP [1]. High doses of MEG (30–60 mg/kg) decreased MAP in normal rats [1]. In two models of acute inflammation, when given at 25 μg/paw in the paw edema model and 10 mg/kg in the pleurisy model, MEG inhibited the inflammatory response [3].
References:
[1] Southan G J, Zingarelli B, O'Connor M, et al. Spontaneous rearrangement of aminoalkylisothioureas into mercaptoalkylguanidines, a novel class of nitric oxide synthase inhibitors with selectivity towards the inducible isoform[J]. British journal of pharmacology, 1996, 117(4): 619-632.
[2] Beck K F, Eberhardt W, Frank S, et al. Inducible NO synthase: role in cellular signalling[J]. Journal of Experimental Biology, 1999, 202(6): 645-653.
[3] Cuzzocrea S, Zingarelli B, Hake P, et al. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation[J]. Free Radical Biology and Medicine, 1998, 24(3): 450-459.
| Physical Appearance | A crystalline solid |
| Storage | Store at 4°C |
| M.Wt | 336.4 |
| Cas No. | 3979-00-8 |
| Formula | [C3H9N3S]2 · H2SO4 |
| Solubility | ≤10mg/ml in PBS(pH7.2) |
| Chemical Name | (2-mercaptoethyl)-guanidine sulfate |
| SDF | Download SDF |
| Canonical SMILES | NC(NCCS)=N.OS(O)(=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure