LXR-623
LXR-623 is an agonist of liver X-receptor with IC50 values of 179nM and 24nM for LXR-α and LXR-β, respectively [1].
LXR-623 is a partial agonist of LXR which regulates the transcription of genes involved in cellular cholesterol homeostasis. As the LXR agonist, LXR-623 is used to enhance reverse cholesterol transport through up-regulating cholesterol transporters. To rodents, orally dosed LXR-623 increases the transcription level of ABCA1 and ABCG1 in peripheral blood cells. To human, LXR-623 also significantly increases ABCA1 and ABCG1 both in PBMC cells and in T- and B-cells. In addition, in mouse model of atherosclerosis, LXR-623 is found to up-regulate intestinal ABCG5 and ABCG8. In cynomolgus monkeys, LXR-623 up-regulates ABCA1 and ABCG1 in whole blood. Besides that, LXR-623 is proposed to be used in treatment of Alzheimer’s disease since it can lower the level of amyloid-βin brain [1, 2].
References:
[1] Katz A, Udata C, Ott E, et al. Safety, Pharmacokinetics, and Pharmacodynamics of Single Doses of LXR‐623, a Novel Liver X‐Receptor Agonist, in Healthy Participants. The Journal of Clinical Pharmacology, 2009, 49(6): 643-649.
[2] DiBlasio-Smith E A, Arai M, Quinet E M, et al. Discovery and implementation of transcriptional biomarkers of synthetic LXR agonists in peripheral blood cells. J Transl Med, 2008, 6: 59.
- 1. Jing Zeng, Daitze Wu, et al. "Activation of the liver X receptor pathway inhibits HBV replication in primary human hepatocytes." Hepatology. 2020 Dec;72(6):1935-1948. PMID: 32145089
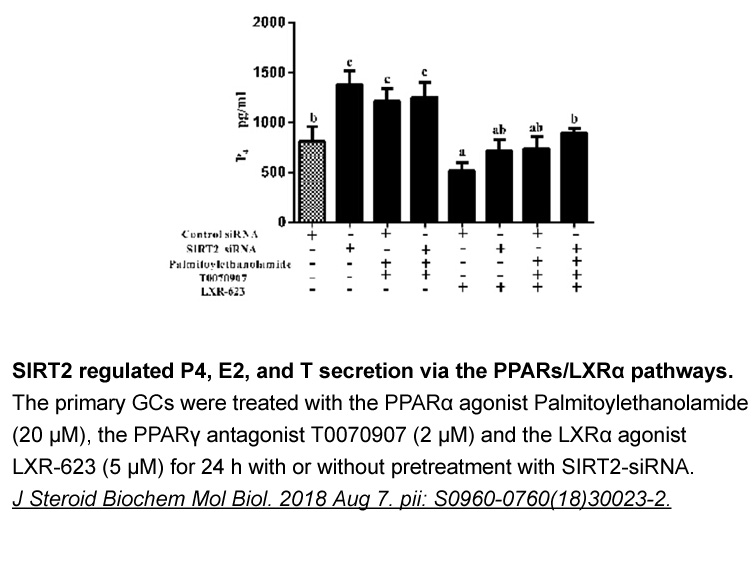
- 2. Xu D, He H, et al. "SIRT2 plays a novel role on progesterone, estradiol and testosterone synthesis via PPARs/LXRα pathways in bovine ovarian granular cells." J Steroid Biochem Mol Biol. 2018 Aug 7. pii: S0960-0760(18)30023-2. PMID: 30009951
- 3. Xian X, Ding Y, et al. "LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis."Elife. 2017 Nov 16;6. pii: e29292. PMID: 29144234
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 422.78 |
| Cas No. | 875787-07-8 |
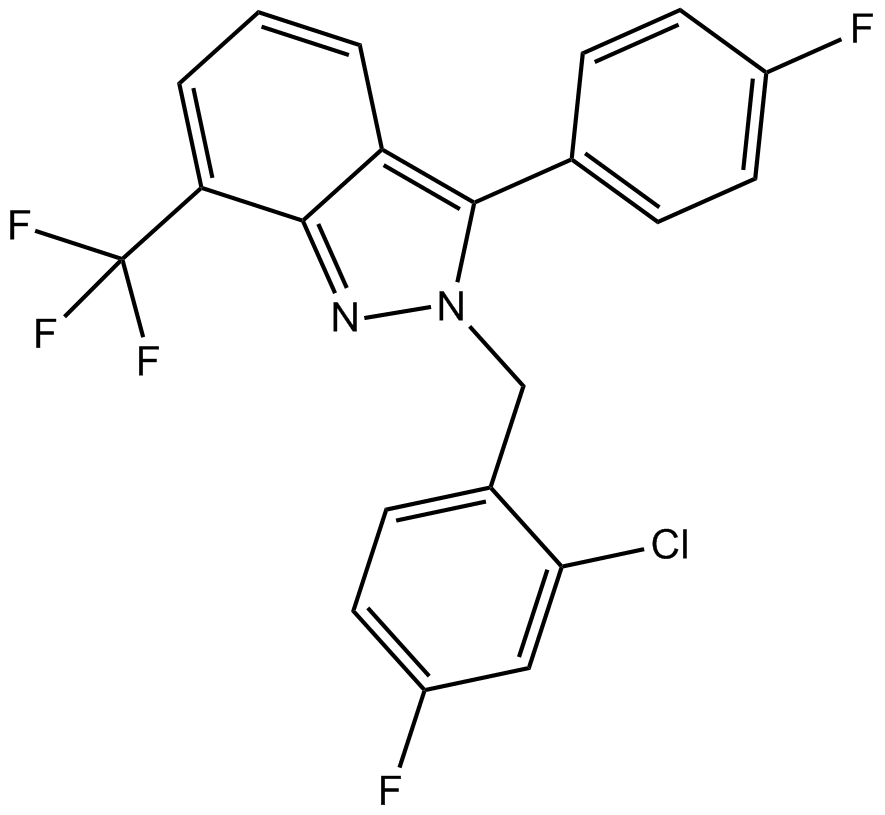
| Formula | C21H12ClF5N2 |
| Solubility | insoluble in H2O; ≥15.49 mg/mL in EtOH with ultrasonic; ≥19.4 mg/mL in DMSO |
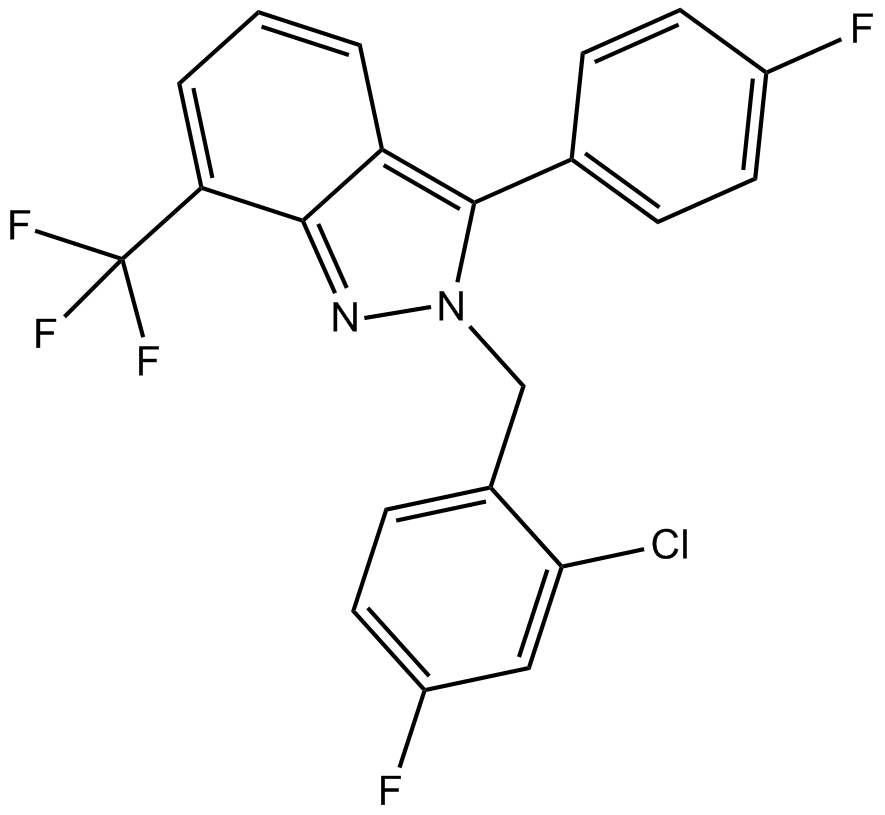
| Chemical Name | 2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)indazole |
| SDF | Download SDF |
| Canonical SMILES | FC(c1cccc2c(-c(cc3)ccc3F)[n](Cc(ccc(F)c3)c3Cl)nc12)(F)F |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Human peripheral blood mononuclear cells |
|
Preparation method |
The solubility of this compound in DMSO is >19.4mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
2 μM, 18 hours |
|
Applications |
LXR-623 (2 μM for either 24 or 48 hours) treatment significantly upregulated mRNA for ABCA1, ABCG1, and PLTP in human PBMC. In PBMC, monocytes, T cells, and B cells, treatment with 2 μM LXR-623 for 18 hours resulted in approximately 6 fold induction of ABCA1 mRNA levels. |
| Animal experiment [1]: | |
|
Animal models |
C57/Bl6 mice, Normal male rats |
|
Dosage form |
Oral administration, 50 mg/kg, 30 mg/kg |
|
Application |
Oral administration of LXR-623 (30 mg/kg) induced ABCA1 and ABCG1 gene expression in rodent peripheral blood cells in vivo. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. DiBlasio-Smith E A, Arai M, Quinet E M, et al. Discovery and implementation of transcriptional biomarkers of synthetic LXR agonists in peripheral blood cells[J]. Journal of translational medicine, 2008, 6(1): 59. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data

Related Biological Data