Lapatinib
Lapatinib (CAS 231277-92-2) is a small-molecule inhibitor targeting the epidermal growth factor receptor (EGFR) and ErbB2 (HER2) tyrosine kinases. It is designed to inhibit these receptors’ kinase activity, thereby blocking autophosphorylation and downstream signaling involved in cell proliferation and survival.
Lapatinib exerts its biological activity primarily through reversible inhibition of EGFR and HER2 tyrosine kinase domains. In cell-free kinase assays, Lapatinib demonstrates potent inhibitory activity, with IC50 values of 10.8 nM for EGFR and 9.2 nM for HER2.
Lapatinib is utilized in cancer and oncology studies.
- 1. Wei Zhang, Jiaqing Lang, et al. "NRF2-mediated persistent adaptation of oesophageal adenocarcinoma cells to HER2 inhibition." Oncogene. 2025 Jun 5;44(33):2929–2941. PMID: 40473905
- 2. Kang-Yao Zeng, Qing-Yi Zhou, et al. "Apoptozole Modulates ABCG2 - Mediated Multidrug Resistance in Lung Cancer." Journal of Cancer Research Updates, 14, 63–68
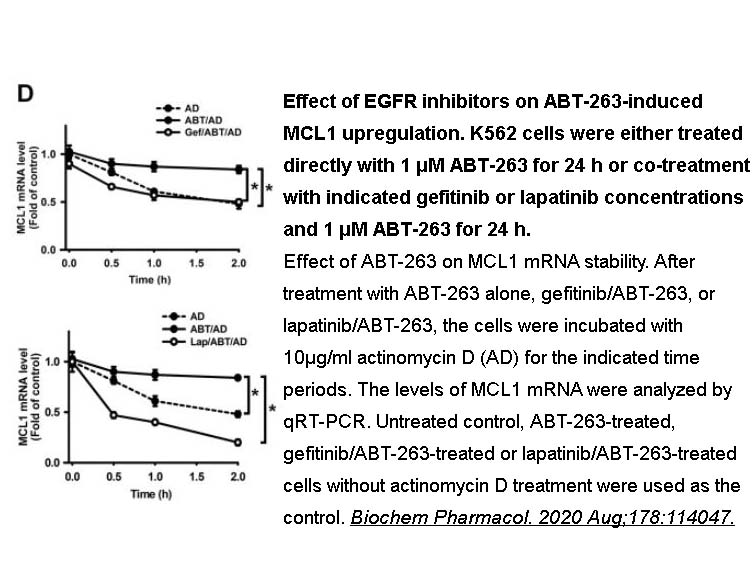
- 3. Lee YC, Wang LJ, et al. "Inhibition of EGFR pathway promotes the cytotoxicity of ABT-263 in human leukemia K562 cells by blocking MCL1 upregulation." Biochem Pharmacol. 2020;178:114047. PMID:32446890
- 4. Ying Z, Beronja S. "Embryonic Barcoding of Equipotent Mammary Progenitors Functionally Identifies Breast Cancer Drivers." Cell Stem Cell. 2020;S1934-5909(20)30009-6. PMID:32059806
- 5. Neal Shah, Pharm.D. "Investigational Chemotherapy and Novel Pharmacokinetics for the Treatment of Cancers of the CNS." West Virginia University. 2019.
- 6. Duggan BM, Foley KP, et al. "Tyrosine kinase inhibitors of Ripk2 attenuate bacterial cell wall-mediated lipolysis, inflammation and dysglycemia." Sci Rep. 2017 May 8;7(1):1578. PMID:28484277
- 7. Zhang WJ, Li Y, et al. "Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer." Cancer Lett. 2017 Feb 1;386:100-109. PMID:27864115
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 581.06 |
| Cas No. | 231277-92-2 |
| Formula | C29H26ClFN4O4S |
| Synonyms | Tykerb;GW572016;GW-572016;GW 572016, Lapatinib tosilate hydrate |
| Solubility | ≥29.05 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
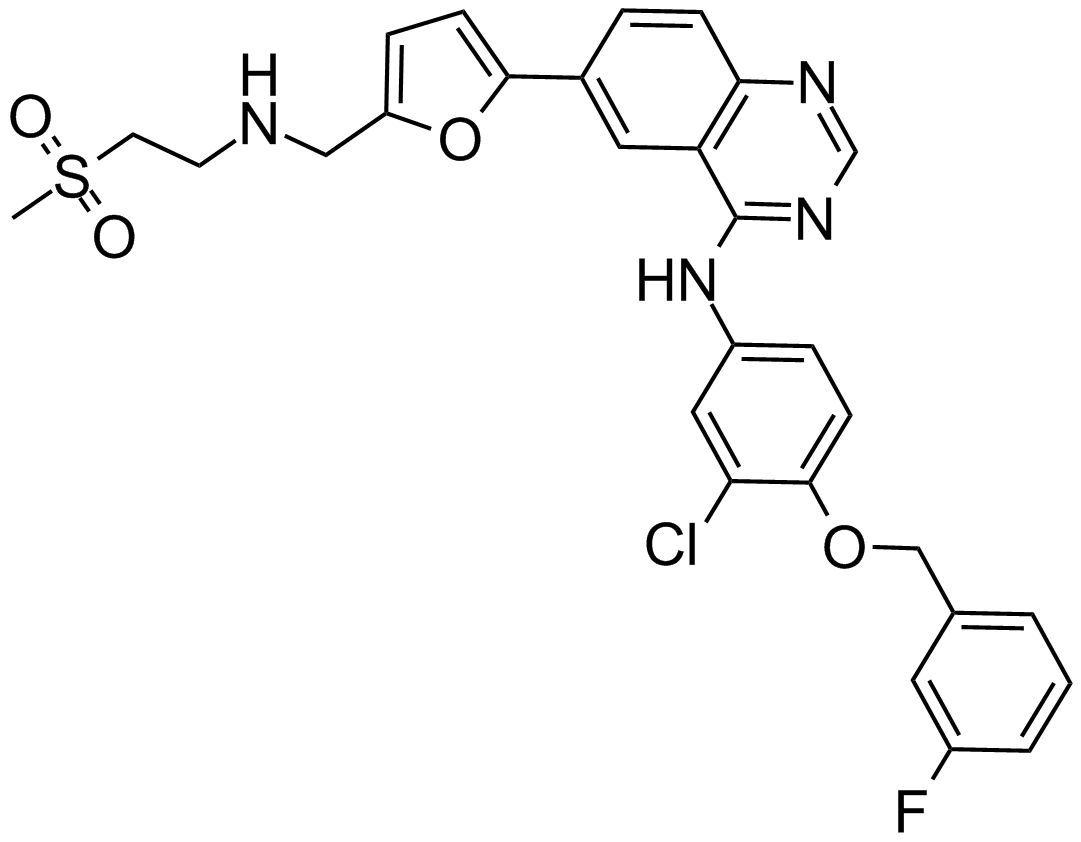
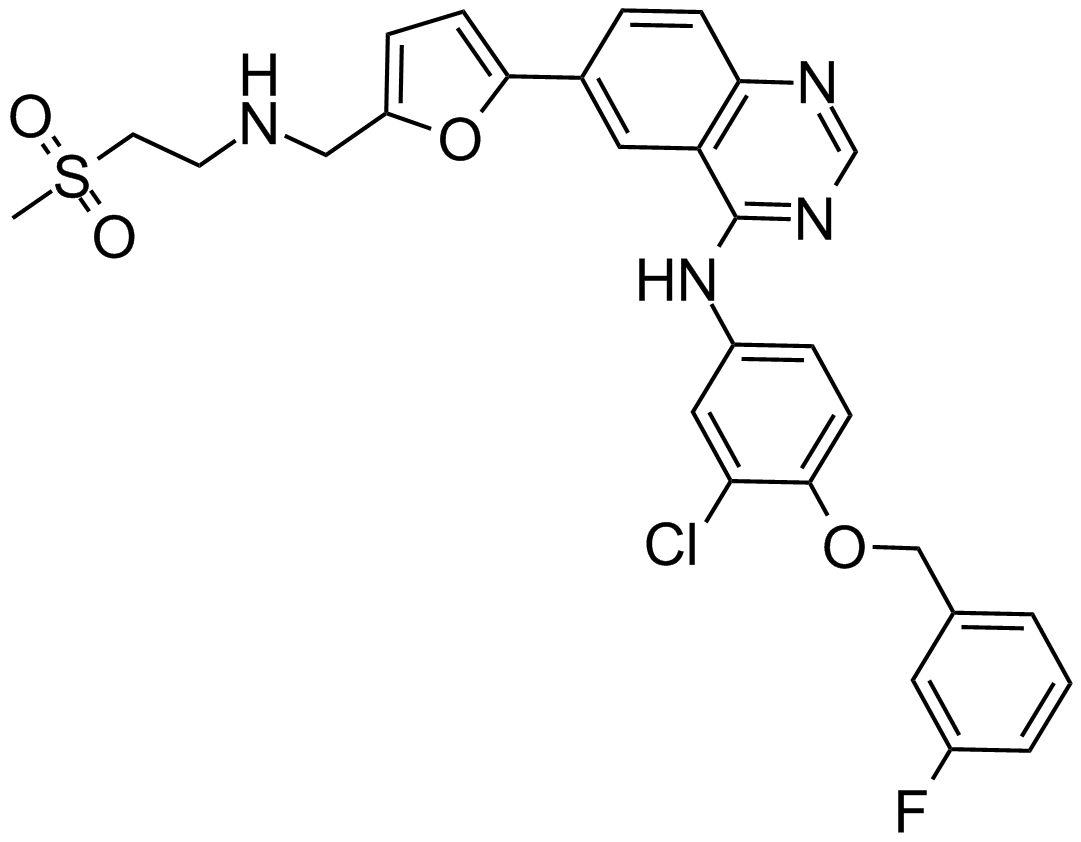
| Chemical Name | N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]furan-2-yl]quinazolin-4-amine |
| SDF | Download SDF |
| Canonical SMILES | CS(=O)(=O)CCNCC1=CC=C(O1)C2=CC3=C(C=C2)N=CN=C3NC4=CC(=C(C=C4)OCC5=CC(=CC=C5)F)Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Binding assays |
The intracellular kinase domains of EGFR, ErbB-2, and ErbB4 were purified from a baculovirus expression system. EGFR, ErbB-2, and ErbB-4 reactions were performed in 96-well polystyrene round-bottomed plates in a final volume of 45 μl. Reaction mixtures contained 50 mM 4-morpholinepropanesulfonic acid (pH 7.5), 2 mM MnCl2, 10 μM ATP, 1 μCi of [γ-33P] ATP/reaction, 50 μM Peptide A [Biotin-(amino hexonoic acid)-EEEEYFELVAKKK-CONH2; Quality Controlled Biochemicals, Inc.], 1 mM dithiothreitol, and 1 μl of DMSO containing serial dilutions of GW2016 beginning at 10 μM. The reaction was initiated by adding the indicated purified type-1 receptor intracellular domain. The amount of enzyme added was 1 pmol/reaction (20 nM). Reactions were terminated after 10 min at 23°C by adding 45 μl of 0.5% phosphoric acid in water. The terminated reaction mix (75 μl) was transferred to phosphocellulose filter plates. The plates were filtered and washed three times with 200 μl of 0.5% phosphoric acid. Scintillation cocktail (50 μl ) was added to each well, and the assay was quantified by counting in a Packard Topcount. |
| Cell experiment [1]: | |
|
Cell lines |
EGFR-overexpressing cell lines HN5 and A-431; the ErbB-2-overexpressing cell lines BT474, N87 (20), and CaLu-3; and tumor cell lines expressing low levels of EGFR and ErbB-2, MCF-7, and T47D |
|
Preparation method |
The solubility of this compound in DMSO is >29.1mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
30 μM, 3 days |
|
Applications |
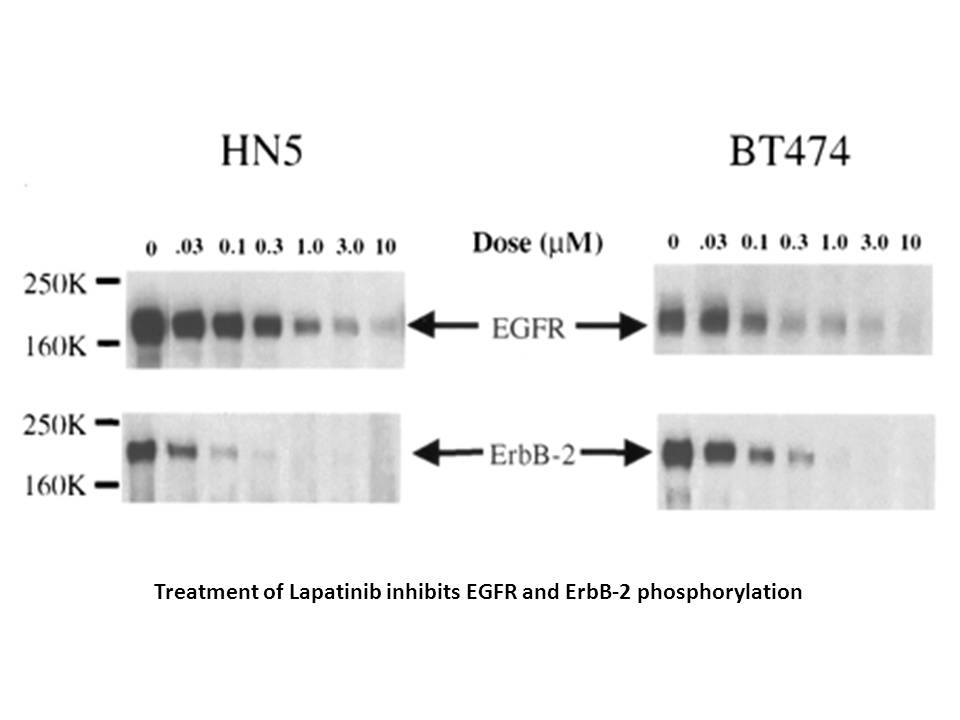
GW2016 (30 μM) resulted in complete inhibition of outgrowth of the HN5 cell population. GW2016 (>3.3 μM) inhibited the outgrowth by 50%. GW2016 (0.37 μM) significantly inhibited the outgrowth by 20%. GW2016 (1 μM) completely inhibited the outgrowth of the BT474 cells, with ~60% inhibition of outgrowth occurring at 0.37 μM. In the EGFR-overexpressing cell line HN5, treatment with GW2016 (1 and 10 μM) resulted in induction of G1 arrest. GW2016 (10 μM for 72 h) slightly increased the number of cells with sub-2N DNA content. In the BT474 cells, a large increase in the number of events with sub-2N DNA was observed after 72 h of treatment with GW2016. |
| Animal experiment [1]: | |
|
Animal models |
BT474 and HN5 human tumor-bearing mice |
|
Dosage form |
Oral administration, 30 and 100 mg/kg, twice daily for 21 days |
|
Application |
Lapatinib (100 mg/kg) completely inhibited tumor growth. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Rusnak D W, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo[J]. Molecular cancer therapeutics, 2001, 1(2): 85-94. |
|
| Description | Lapatinib is a potent inhibitor of EGFR and ErbB2 with IC50 of 10.8 and 9.2 nM, respectively. | |||||
| Targets | EGFR | ErbB2 | ||||
| IC50 | 10.8 nM | 9.2 nM | ||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
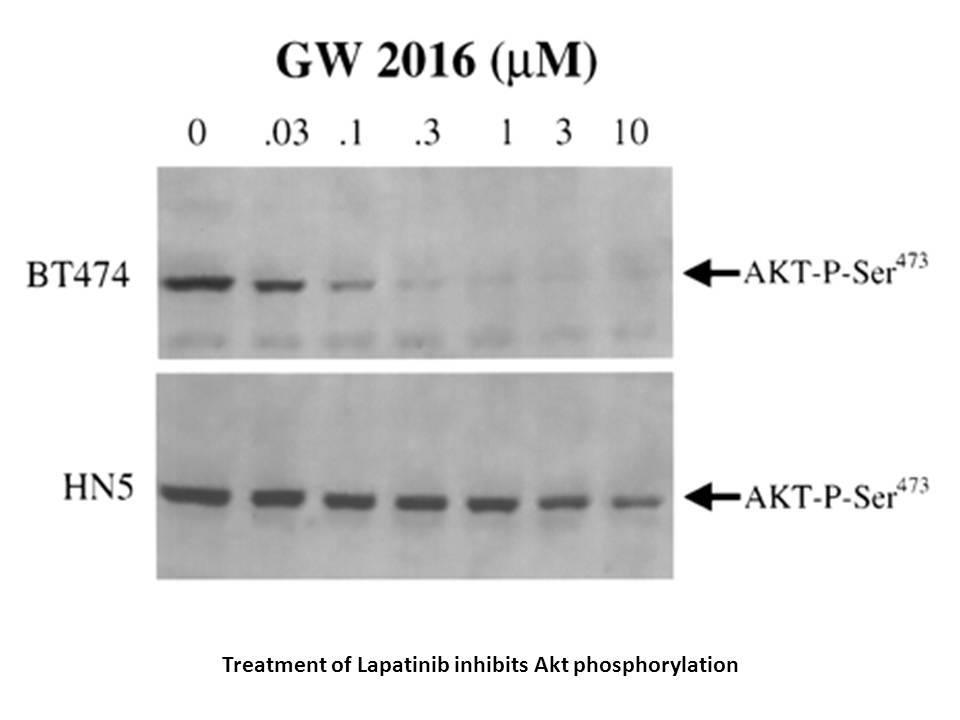
Related Biological Data
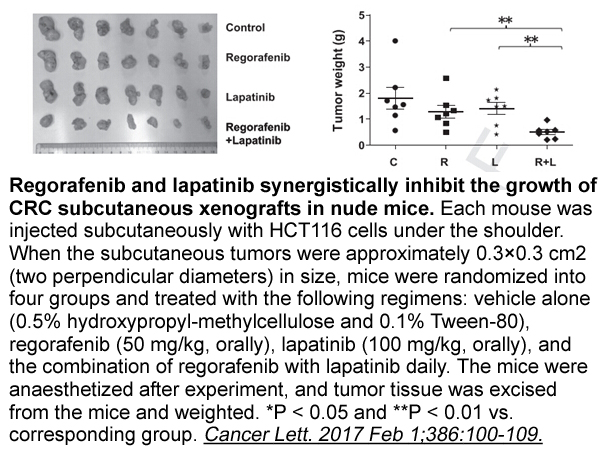
Related Biological Data
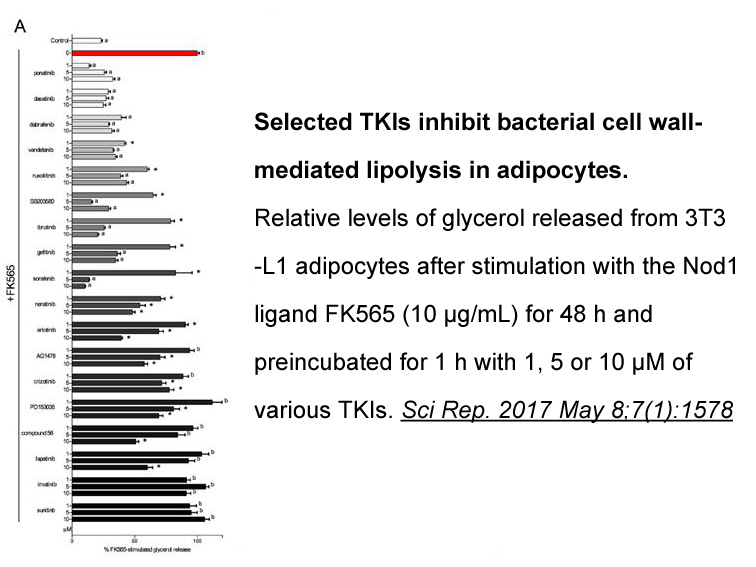
Related Biological Data
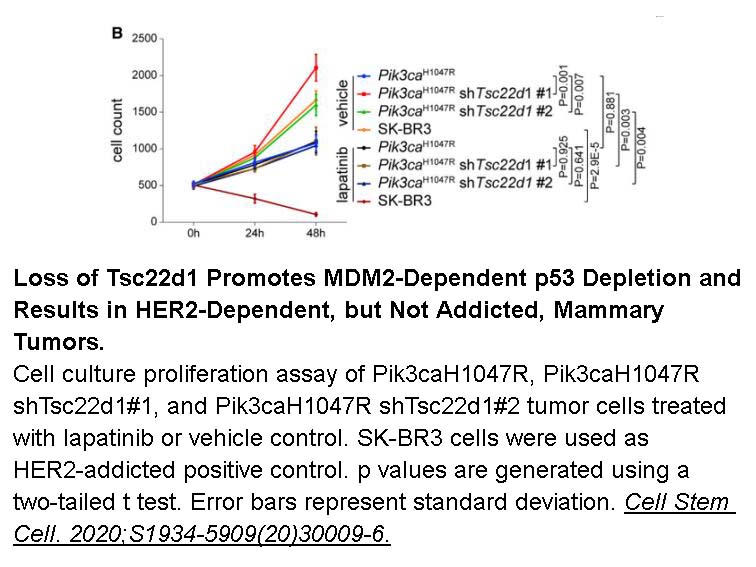
Related Biological Data