Lamivudine
Lamivudine, a nucleoside analog, is a potent reverse transcriptase inhibitor, with IC50 value of 0.316 µM against human immunodeficiency virus type 1 (HIV-1) [1].
Reverse transcriptase is a RNA-dependent DNA polymerase, playing a critical role in retroviral replication. Thus, reverse transcriptase has been made a target of antiretroviral chemotherapy [1].
Lamivudine was most potent against simian retrovirus types 1 and 2 (SRV-1, SRV-2), and HIV-1, with IC50 values of 10, 0.316 and 0.316 µM, respectively, but did not inhibit foamy viruses and amphotrophic murine leukaemia virus (MLV-A) [1].
In human coinfected with HIV-1 and hepatitis B virus (HBV), Lamivudine (150 mg, b.i.d., p.o.) displayed dual efficacy through both delayed HIV-1 disease progression and a favorable biochemical and HBV virologic response. Twelve months of Lamivudine therapy led to HBV DNA and hepatitis B e antigen (HBeAg) losses of ∼40% and ∼20%, respectively, and more alanine aminotransferase (ALT) normalization than that in the placebo group [2].
References:
[1]. Rosenblum L L, Patton G, Grigg A R, et al. Differential susceptibility of retroviruses to nucleoside analogues. Antiviral Chemistry and Chemotherapy, 2001, 12(2): 91-97.
[2]. Dore G J, Cooper D A, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. The Journal of Infectious Diseases, 1999, 180(3): 607-613.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 229.26 |
| Cas No. | 134678-17-4 |
| Formula | C8H11N3O3S |
| Solubility | ≥10.5 mg/mL in DMSO; ≥11.4 mg/mL in EtOH with ultrasonic; ≥89.4 mg/mL in H2O with ultrasonic |
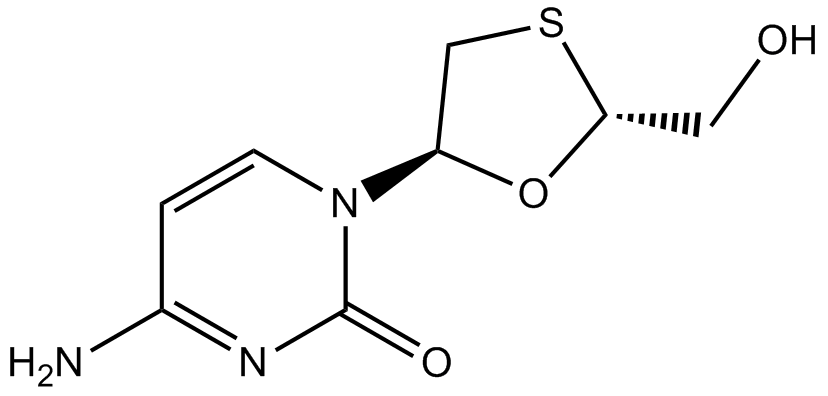
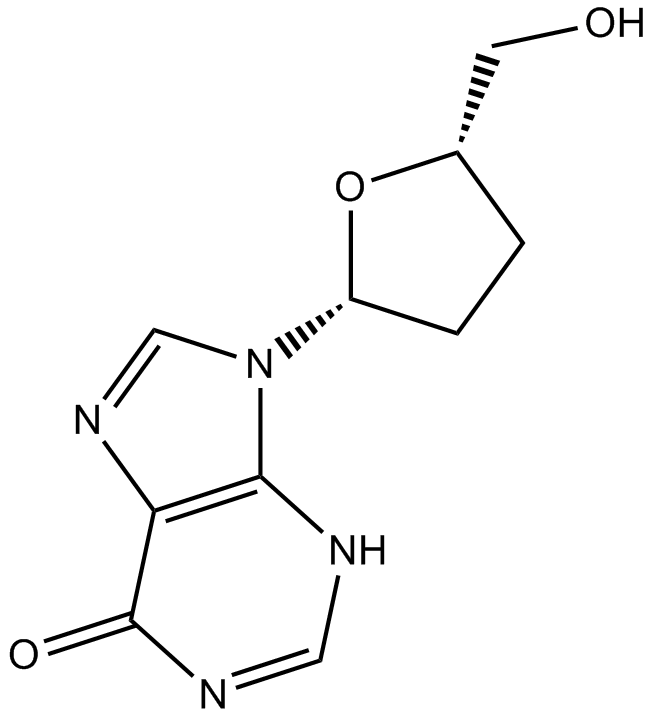
| Chemical Name | 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one |
| SDF | Download SDF |
| Canonical SMILES | NC(C=CN1[C@H]2O[C@@H](CO)SC2)=NC1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
Peripheral blood mononuclear cells (PBMCs) |
|
Reaction Conditions |
10 d incubation |
|
Applications |
Lamivudine inhibited p24 antigen production by HIV-I in PBMCs, with ED50s ranging from 0.07 to 0.2 μM. There was no toxicity in uninfected PBMC with concentrations of lamivudine up to 10 μM over a 10-day culture period. |
| Animal experiment:[2] | |
|
Animal models |
Humanized BALB/c Rag1-/-γc-/- or Rag2-/-γc-/- mice |
|
Dosage form |
7 mg drug tablet containing lamivudine, abacavir and dolutegravir Re-suspended in 100 μl distilled water for daily gavage |
|
Applications |
Oral drug treatment led to full viral suppression and protection from CD4 T cell depletion. Cessation of therapy resulted in viral rebound and CD4 T cell loss. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Merrill DP, Moonis M, Chou TC, et al. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type 1 replication in vitro. The Journal of Infectious Diseases, 1996, 173(2): 355-364. 2. Hu S, Neff CP, Kumar DM, et al. A humanized mouse model for HIV-2 infection and efficacy testing of a single-pill triple-drug combination anti-retroviral therapy. Virology, 2017, 501: 115-118. |
|
Quality Control & MSDS
- View current batch:
Chemical structure