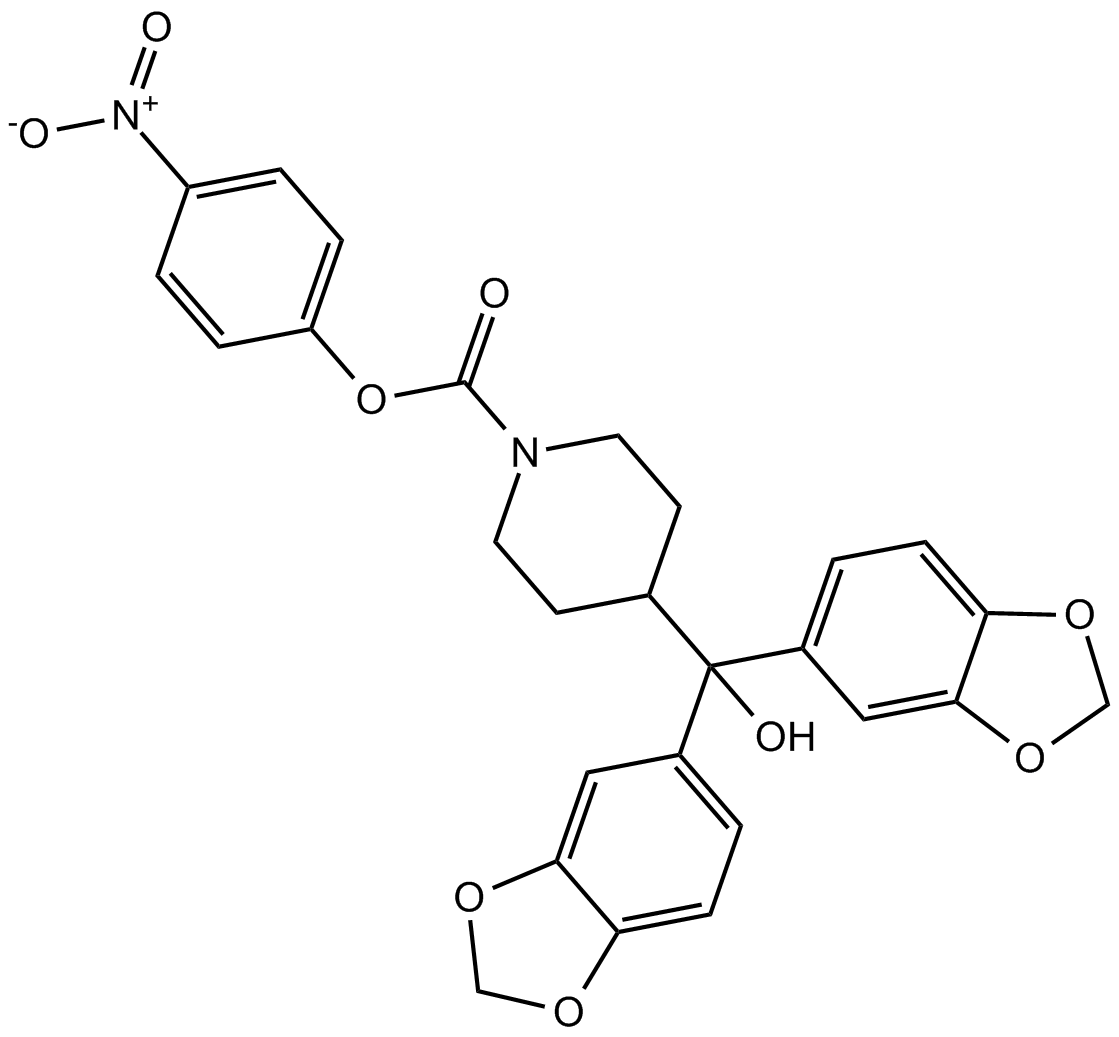
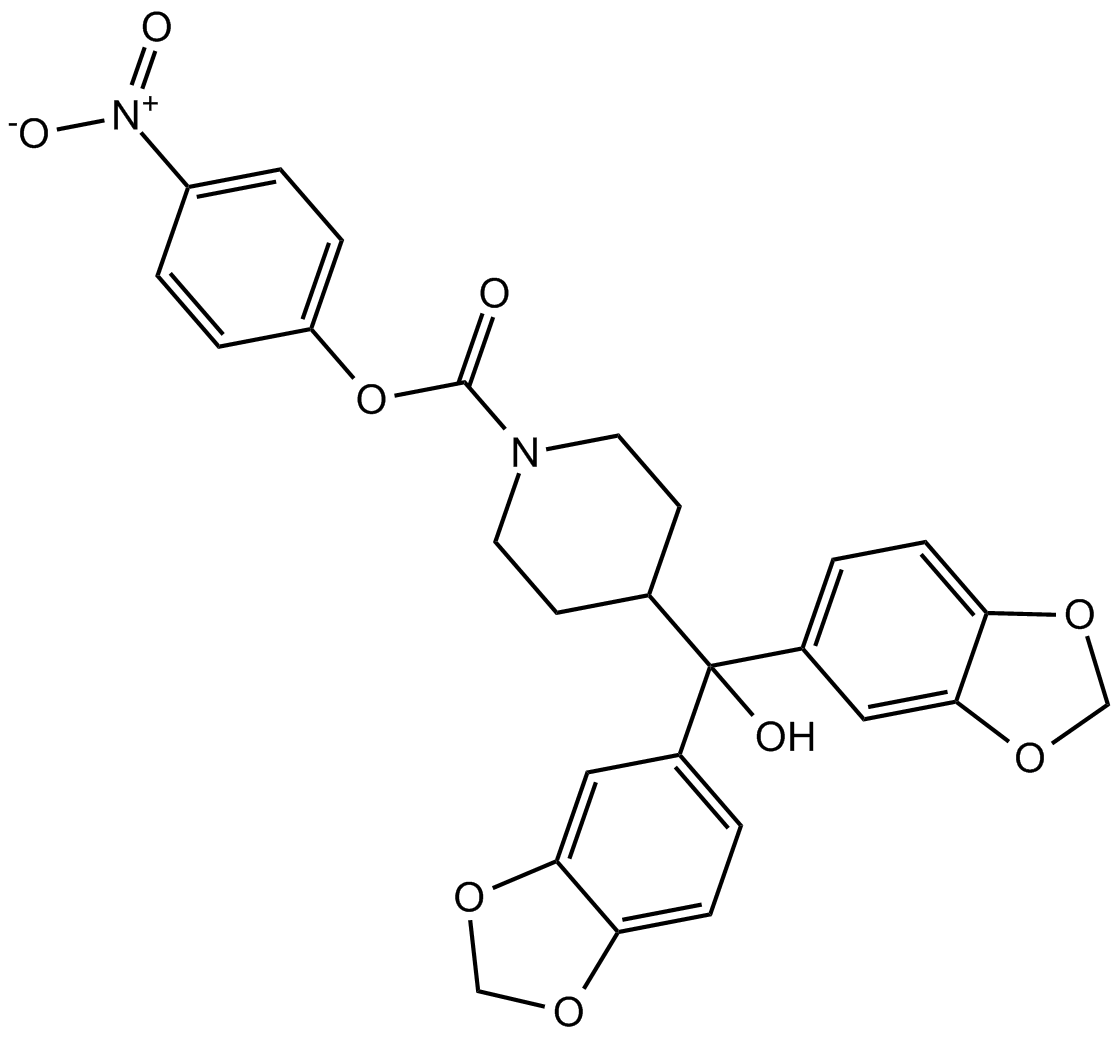
JZL184
JZL184 is a potent and selective inhibitor of MAGL [1].
Monoacylglycerol lipase (MAGL) is a membrane-associated member of the serine hydrolase superfamily and hydrolyzes endocannabinoid 2-arachidonoylglycerol (2-AG) and intracellular triglyceride stores.
JZL184 is a potent and selective MAGL inhibitor. In cerebellar Purkinje neurons, JZL184 (40-120 min) prolonged depolarization-induced suppression of excitation (DSE) in a dose-dependent way, which was mediated by CB1 receptor. In hippocampal CA1 pyramidal neurons, JZL184 (100 nM) significantly prolonged depolarization-induced suppression of inhibition (DSI), which was mediated by CB1 receptor activation. In mouse cerebellar slices, JZL184 (100 nM) significantly enhanced 2-AG (10 μM)-induced depression of EPSCs in Purkinje neurons. These results suggested that MAGL is the primary mechanism by which 2-AG is metabolized [1].
In mice, JZL184 inhibited 2-AG hydrolysis with IC50 value of 8 nM and increased brain 2-AG by 8-fold. The JZL184-treated mice exhibited CB1-dependent behaviors including analgesia, hypomotility and hypothermia [2]. In rats, JZL184 (8 mg/kg) exhibited anxiolytic-like effects via inhibition of MGL mediated 2-AG hydrolysis under high levels of environmental aversiveness [3]. In rats, JZL184 produced antinociception with ED50 values of 0.06 and 0.03 μM in the early phase and the late phase of formalin pain, respectively [4].
References:
[1]. Pan B, Wang W, Long JZ, et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther, 2009, 331(2): 591-597.
[2]. Long JZ, Li W, Booker L, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol, 2009, 5(1): 37-44.
[3]. Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res, 2011, 64(3): 226-234.
[4]. Guindon J, Guijarro A, Piomelli D, et al. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br J Pharmacol, 2011, 163(7): 1464-1478.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 520.49 |
| Cas No. | 1101854-58-3 |
| Formula | C27H24N2O9 |
| Solubility | insoluble in H2O; insoluble in EtOH; ≥20.35 mg/mL in DMSO |
| Chemical Name | (4-nitrophenyl) 4-[bis(1,3-benzodioxol-5-yl)-hydroxymethyl]piperidine-1-carboxylate |
| SDF | Download SDF |
| Canonical SMILES | C1CN(CCC1C(C2=CC3=C(C=C2)OCO3)(C4=CC5=C(C=C4)OCO5)O)C(=O)OC6=CC=C(C=C6)[N+](=O)[O-] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure