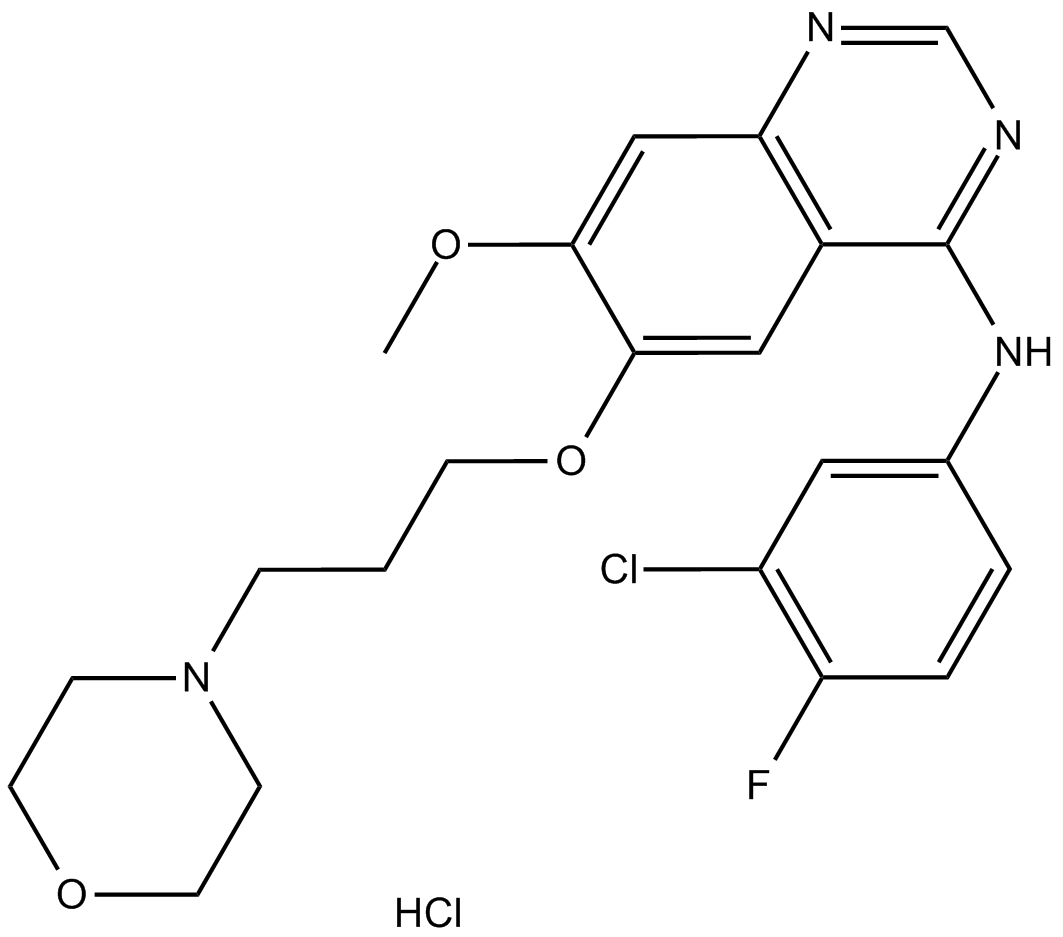
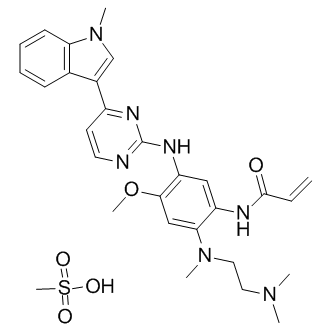
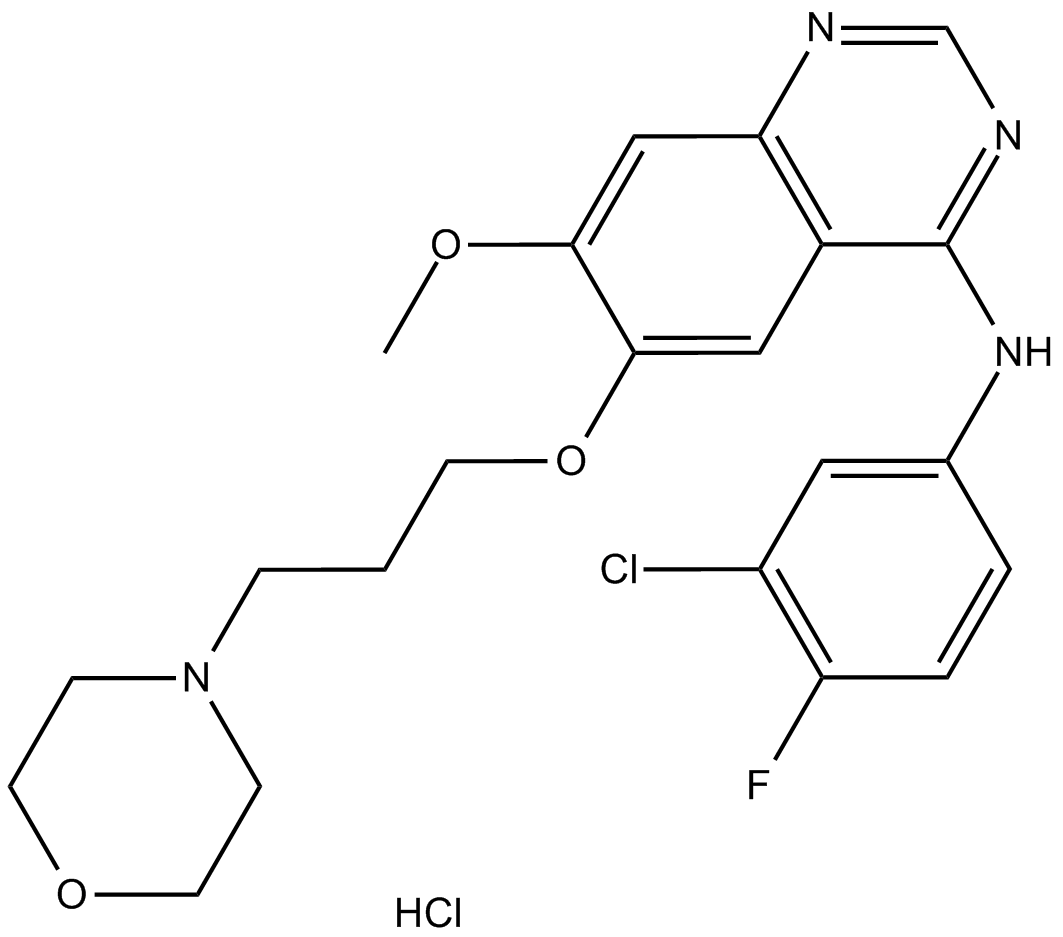
Gefitinib hydrochloride
The EGFR is a Mr 170,000 transmembrane glycoprotein with an external binding domain and an intracellular tyrosine kinase domain. Gefitinib (ZD-1839, Iressa) is an Epidermal Growth Factor Receptor-selective Tyrosine Kinase Inhibitor.
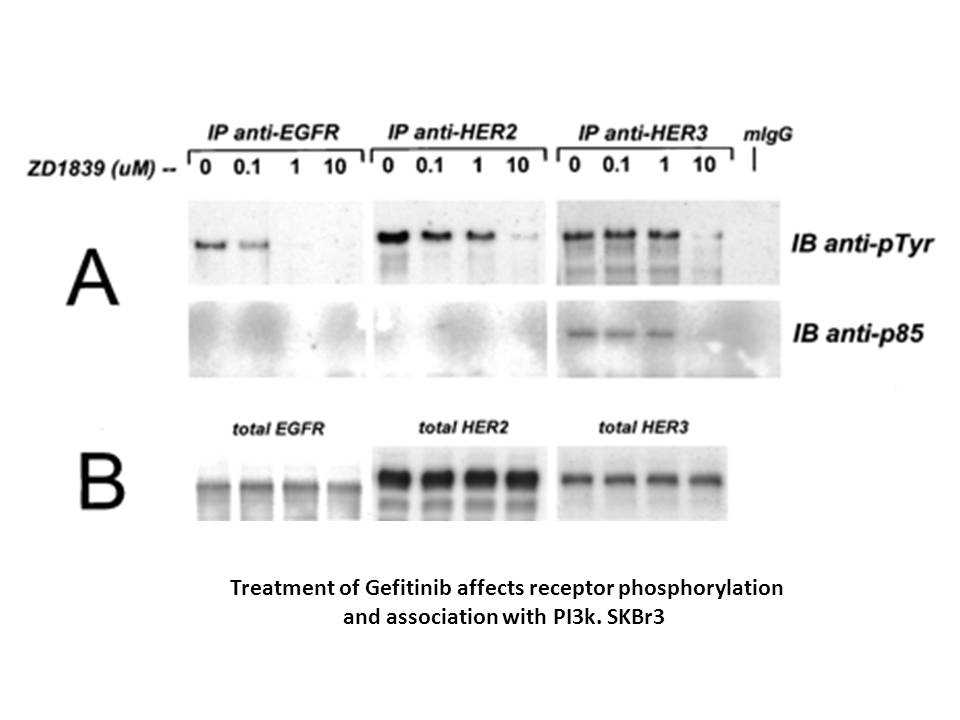
In vitro: Gefitinib inhibited colony formation in soft agar in a dose dependent manner in all cancer cell lines. However, treatment with higher doses resulted in a 2–4-fold increases in apoptosis. Dose-dependent supra-additive increase in growth inhibition was observed when cancer cells were treated with totoxic drugs and Gefitinib. The combined treatment markedly enhanced apoptotic cell death induced by single agent treatment [1].
In vivo: Gefitinib treatment of nude mice bearing established human GEO colon cancer xenografts revealed a reversible dose-dependent inhibition of tumor growth because GEO tumors resumed the growth rate of controls at the end of the treatment [1].
Clinical trial: Administration of a 250-mg dose of gefitinib as a dispersion preparation by drink or nasogastric tube achieved a systemic exposure to gefitinib that was consistent with that achieved when gefitinib was administered as a whole tablet. No evidence of tolerability problems associated with the routes of administration studied was observed in these healthy volunteers [2].
References:
[1] Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6(5):2053-63.
[2] Cantarini MV, McFarquhar T, Smith RP, Bailey C, Marshall AL. Relative bioavailability and safety profile of gefitinib administered as a tablet or as a dispersion preparation via drink or nasogastric tube: results of a randomized, open-label, three-period crossover study in healthy volunteers. Clin Ther. 2004;26(10):1630-6.
| Storage | Store at -20°C |
| M.Wt | 483.36 |
| Cas No. | 184475-55-6 |
| Formula | C22H25Cl2FN4O3 |
| Synonyms | Iressa hydrochloride;ZD 1839 hydrochloride;ZD-1839 hydrochloride;ZD1839 hydrochloride |
| Solubility | insoluble in EtOH; ≥4.28 mg/mL in H2O with gentle warming and ultrasonic; ≥6.9 mg/mL in DMSO with gentle warming |
| Chemical Name | N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine;hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | COc(c(OCCCN1CCOCC1)c1)cc2c1c(Nc(cc1)cc(Cl)c1F)ncn2.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
Human ovarian (OVCAR-3), breast (ZR-75–1, MCF-10A ras), and colon cancer (GEO) cells that coexpress EGFR and TGF-α |
|
Reaction Conditions |
0.01 ~ 50 μM gefitinib for 5 d incubation |
|
Applications |
Gefitinib (0.01 ~ 50 μM; 5 d) inhibited colony formation in soft agar in a dose-dependent manner in all cancer cell lines. The antiproliferative effect was mainly cytostatic. However, treatment with higher doses (0.05, 0.1 or 1 μM; 3 d) resulted in a 2 ~ 4-fold increase in apoptosis. |
| Animal experiment:[1] | |
|
Animal models |
Nude mice bearing established human GEO colon cancer xenografts |
|
Dosage form |
1.25, 2.5 or 5 mg/dose Administered intraperitoneally on days 1 ~ 5 of each week for 4 weeks |
|
Applications |
Gefitinib treatment produced a dose-dependent inhibition of GEO tumor growth that was almost completely suppressed in mice treated with the 5 mg of daily dose. Gefitinib treatment was generally well tolerated by mice with no signs of acute or delayed toxicity. Although GEO tumor growth was only delayed, mouse survival duration was significantly increased in the gefitinib (5 mg/dose)-treated group. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clinical Cancer Research, 2000, 6(5): 2053-2063. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data