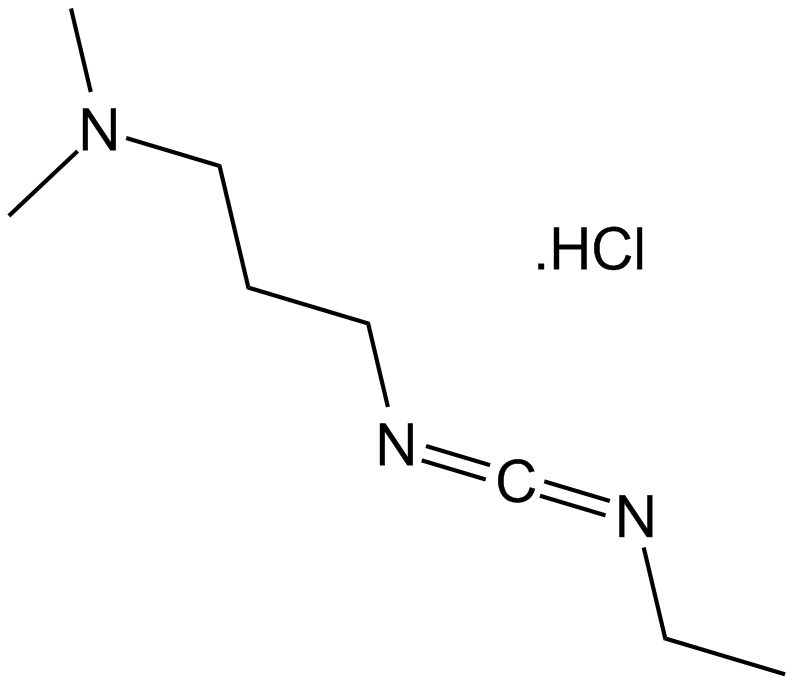
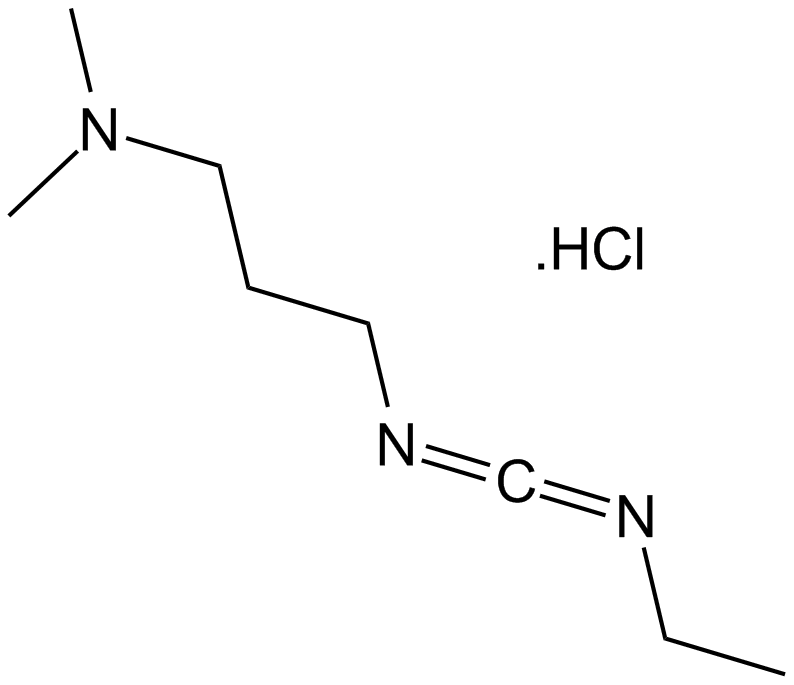
EDC.HCl
EDC.HCl (CAS 25952-53-8) is a water-soluble carbodiimide reagent commonly employed in peptide synthesis and other bioconjugation reactions. It facilitates amide bond formation by activating carboxyl groups, enabling their coupling with primary amines in aqueous media. The coupling mechanism involves initial activation of the carboxylic acid by EDC.HCl, generating an unstable intermediate that reacts readily with amines to produce amide linkages, and ultimately EDC is converted into a urea byproduct. Besides peptide synthesis, the reagent is also utilized for nucleotide synthesis, esterification, and lactonization reactions. EDC.HCl can be quantitatively monitored via spectrophotometric methods. Currently, no in vivo or clinical data are reported.
- 1. Asila Osman, Young Hoon Song, et al. "Sugar Nanocluster Adhesive Boosts Wound Healing in Diabetic Mice." Carbohydrate Polymer Technologies and Applications. Volume 11, September 2025, 100933.
- 2. Min Hee Lee, Seung Ju Choi, et al. "Proteomics and RNA sequencing analysis of Escherichia coli biofilm on reverse osmosis membrane for wastewater reclamation." Desalination. Volume 607, 15 July 2025, 118814
- 3. Xiudan Zheng, Rui Huang, et al. "Injectable antioxidant hyaluronan/chitosan hydrogel as a platelet-rich plasma and stem cell carrier to promote endometrial regeneration and fertility restoration." Acta Biomater. 2025 Mar15:195:201-215 PMID: 39894327
- 4. Cuiping Jiang, Yuan Wang, et al. "ATP-Responsive Multifunctional Supramolecular Polymer as a Nonviral Vector for Boosting Cholesterol Removal from Lipid-Laden Macrophages." ACS Biomater Sci Eng. 2021 Nov 8;7(11):5048-5063. PMID: 34648280
- 5. Zhuxian Wang, Yaqi Xue, et al. "Glycyrrhiza acid micelles loaded with licochalcone A for topical delivery: Co-penetration and anti-melanogenic effect." Eur J Pharm Sci. 2021 Dec 1;167:106029. PMID: 34601069
- 6. Rafiei A, Schriemer DC. "A microtubule crosslinking protocol for integrative structural modeling activities." Anal Biochem. 2019 Sep 6:113416. PMID: 31499019
| Physical Appearance | A solid |
| Storage | Desiccate at -20°C |
| M.Wt | 191.7 |
| Cas No. | 25952-53-8 |
| Formula | C8H17N3·HCl |
| Solubility | ≥19.2 mg/mL in DMSO; ≥39 mg/mL in H2O; ≥39.6 mg/mL in ETOH |
| Chemical Name | 3-(ethyliminomethylideneamino)-N,N-dimethylpropan-1-amine;hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | CCN=C=NCCCN(C)C.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure