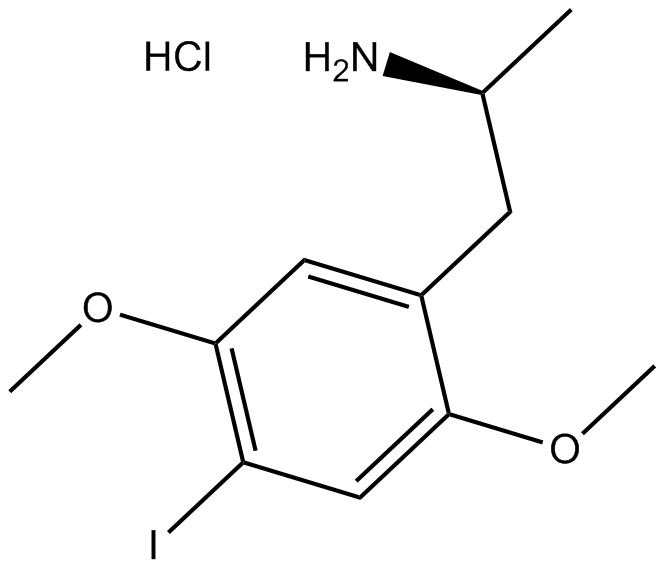
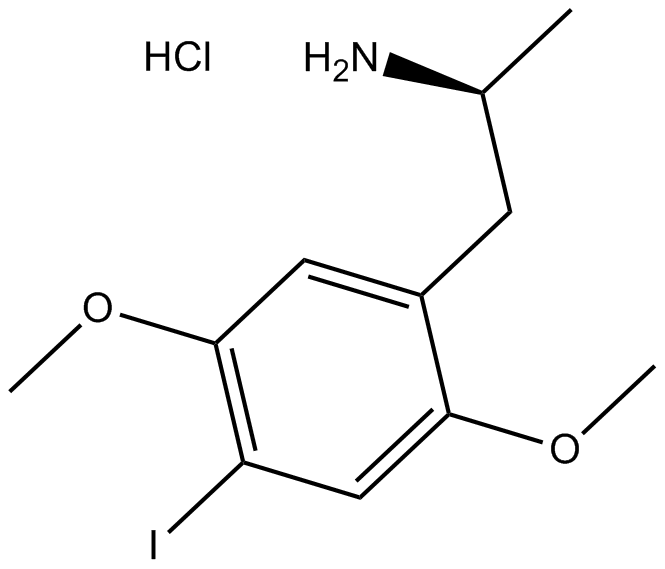
DOI hydrochloride
DOI hydrochloride is a brain-permeable agonist of 5-HT2A and 5-HT2C receptors, with Ki values being 0.7, 2.4 and 20 nM for 5-HT2A, 5-HT2C and 5-HT2B receptors, respectively. The 5-HT2 receptor consists of three distinct subpopulations, i.e. 5-HT2A, 5-HT2B, and 5-HT2C receptors, among which the 5-HT2A receptor contributes to the major part of the behavioral effects evoked by hallucinogens. DOI, as a phenylisopropylamine hallucinogen, can thus be used to explore 5-HT2 receptor mediated biological events due to its high affinity for the 5-HT2 receptor. In addition, DOI has also been used as an analytical reference standard categorized as a phenethylamine and amphetamine.
References:
1. Nelson DL, Lucaites VL, Wainscott DB, et al. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn-Schmiedeberg's Archives of Pharmacology, 1999, 359(1): 1-6.
2. Kerrigan S, Banuelos S, Perrella L, et al. Simultaneous detection of ten psychedelic phenethylamines in urine by gas chromatography-mass spectrometry. Journal of Analytical Toxicology, 2011, 35(7): 459-469.
3. Yu B, Becnel J, Zerfaoui M, et al. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. Journal of Pharmacology and Experimental Therapeutics, 327(2): 316-323.
4. Monti JM, Jantos H. Effects of the serotonin 5-HT2A/2C receptor agonist DOI and of the selective 5-HT2A or 5-HT2C receptor antagonists EMD 281014 and SB-243213, respectively, on sleep and waking in the rat. European Journal of Pharmacology, 2006, 553(1-3): 163-170.
| Physical Appearance | A crystalline solid |
| Storage | Store at RT |
| M.Wt | 357.62 |
| Cas No. | 42203-78-1 |
| Formula | C11H16INO2·HCl |
| Solubility | Soluble in H2O |
| Chemical Name | (S)-1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | C[C@@H](Cc(c(OC)c1)cc(OC)c1I)N.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[3] | |
|
Cell lines |
Rat aortic smooth muscle (RASM) cells |
|
Reaction Conditions |
1 nM DOI |
|
Applications |
DOI rapidly blocked TNF-α-induced ICAM1 expression in RASM cells. Moreover, the effect of DOI at blocking proinflammatory gene expression remained many hours after addition of TNF-α. |
| Animal experiment:[4] | |
|
Animal models |
Male Wistar rats, 350–400 g |
|
Dosage form |
0.35 ~ 0.7 mmol/kg Subcutaneous administration |
|
Applications |
DOI significantly increased waking and light sleep, and reduced slow wave sleep, rapid-eye-movement (REM) sleep, and the number of REM periods in rats. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Nelson DL, Lucaites VL, Wainscott DB, et al. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn-Schmiedeberg's Archives of Pharmacology, 1999, 359(1): 1-6. 2. Kerrigan S, Banuelos S, Perrella L, et al. Simultaneous detection of ten psychedelic phenethylamines in urine by gas chromatography-mass spectrometry. Journal of Analytical Toxicology, 2011, 35(7): 459-469. 3. Yu B, Becnel J, Zerfaoui M, et al. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. Journal of Pharmacology and Experimental Therapeutics, 327(2): 316-323. 4. Monti JM, Jantos H. Effects of the serotonin 5-HT2A/2C receptor agonist DOI and of the selective 5-HT2A or 5-HT2C receptor antagonists EMD 281014 and SB-243213, respectively, on sleep and waking in the rat. European Journal of Pharmacology, 2006, 553(1-3): 163-170. |
|
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure