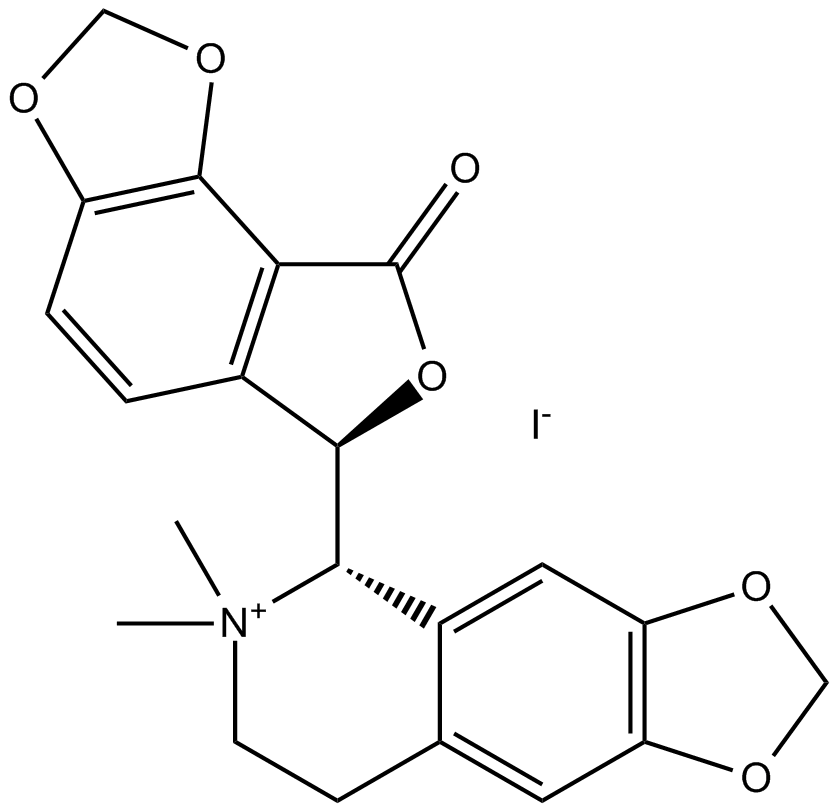
(-)-Bicuculline methiodide
(-)-Bicuculline methiodide (CAS: 40709-69-1) is an antagonist of the γ-aminobutyric acid-A (GABA_A) receptor, inhibiting GABA binding with an IC_50 of approximately 500 μM. GABA_A receptors are ligand-gated chloride ion channels mediating inhibitory neurotransmission in the central nervous system. In studies using chick cerebellar synaptic membranes, this enantiomer exhibited lower activity relative to the (+)-enantiomer. Additionally, in *C. elegans*, (-)-bicuculline methiodide helped demonstrate pharmacological distinctions between UNC-49 GABA receptors and mammalian GABA_A receptors. This compound is employed widely in neuropharmacological research exploring GABA receptor function and regulation.
| Physical Appearance | Yellow solid |
| Storage | Store at RT |
| M.Wt | 509.3 |
| Cas No. | 40709-69-1 |
| Formula | C21H20INO6 |
| Solubility | <10.19mg/ml in H2O; <25.46mg/ml in DMSO |
| Chemical Name | (S)-6,6-dimethyl-5-((R)-8-oxo-6,8-dihydro-[1,3]dioxolo[4,5-e]isobenzofuran-6-yl)-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-6-ium iodide |
| SDF | Download SDF |
| Canonical SMILES | O=C1O[C@H](C(C=C2)=C1C3=C2OCO3)[C@H](C4=C5)[N+](C)(C)CCC4=CC6=C5OCO6.[I-] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure