ATB-343
ATB-343, also named as indomethacin, is an H2S-releasing derivative of indomethacin [1].
Hydrogen sulfide (H2S) is a newly recognized signaling molecule with potent cytoprotective actions. Hydrogen sulfide has been involved in mediating many physiological processes, such as the maintenance of gastrointestinal mucosal defense and repair. Hydrogen sulfide also exerts many anti-inflammatory effects, including inhibition of leukocyte adherence to the vascular endothelium and leukocyte migration to sites of inflammation [2].
In vitro: Indomethacin enhanced the NK cell activity in vitro by blocking the prostaglandin production of monocytes [3].
In vivo: In rats, oral administration of ATB-343 significantly inhibited gastric prostaglandin synthesis and systemic COX-1 activity [1]. Indomethacin was a potent inhibitor of prostaglandin synthesis. Indomethacin suppressed in vivo thyroid hormone secretion and cholera toxin-induced intestinal fluid accumulation. Indomethacin in submicromolar concentrations inhibited cyclic AMP-dependent protein kinase and endogenous protein phosphorylation [4].
Clinical trials: Indomethacin has entered clinical trial in Alzheimer's disease [5].
References:
[1] Wallace J L. Hydrogen sulfide-releasing anti-inflammatory drugs[J]. Trends in Pharmacological Sciences, 2007, 28(10): 501-505.
[2] Wallace J L. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract[J]. Antioxidants & redox signaling, 2010, 12(9): 1125-1133.
[3] Pedersen B K, Oxholm P, Klarlund K. Characterization of the in vivo and in vitro effects of indomethacin on human natural killer cell activity[J]. Allergy, 1986, 41(7): 532-536.
[4] Kantor H S, Hampton M. Indomethacin in submicromolar concentrations inhibits cyclic AMP-dependent protein kinase[J]. 1978.
[5] Rogers J, Kirby L C, Hempelman S R, et al. Clinical trial of indomethacin in Alzheimer's disease[J]. Neurology, 1993, 43(8): 1609-1609.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 566.1 |
| Cas No. | 1000700-26-4 |
| Formula | C28H20ClNO4S3 |
| Solubility | ≤10mg/ml in DMSO;10mg/ml in dimethyl formamide |
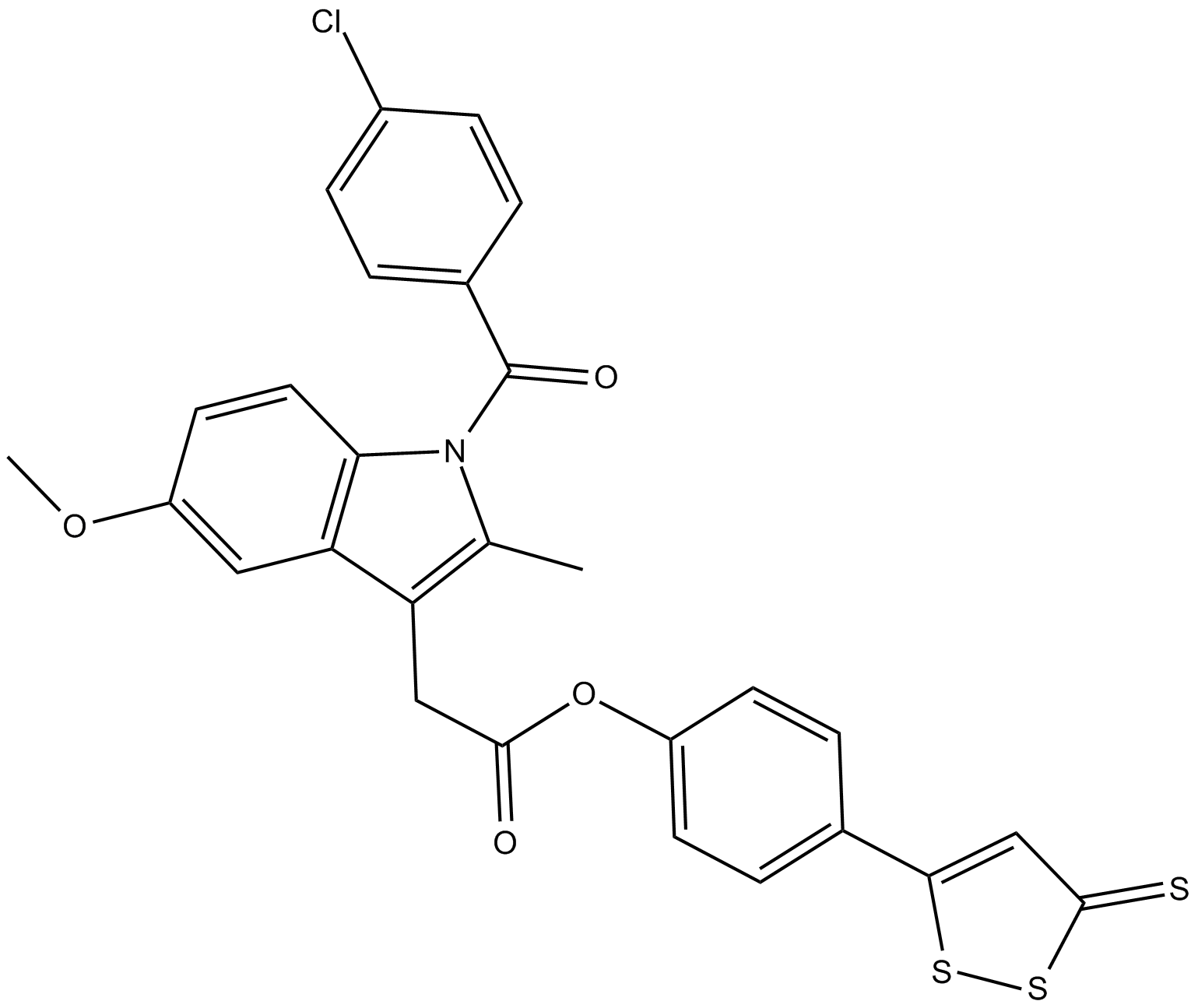
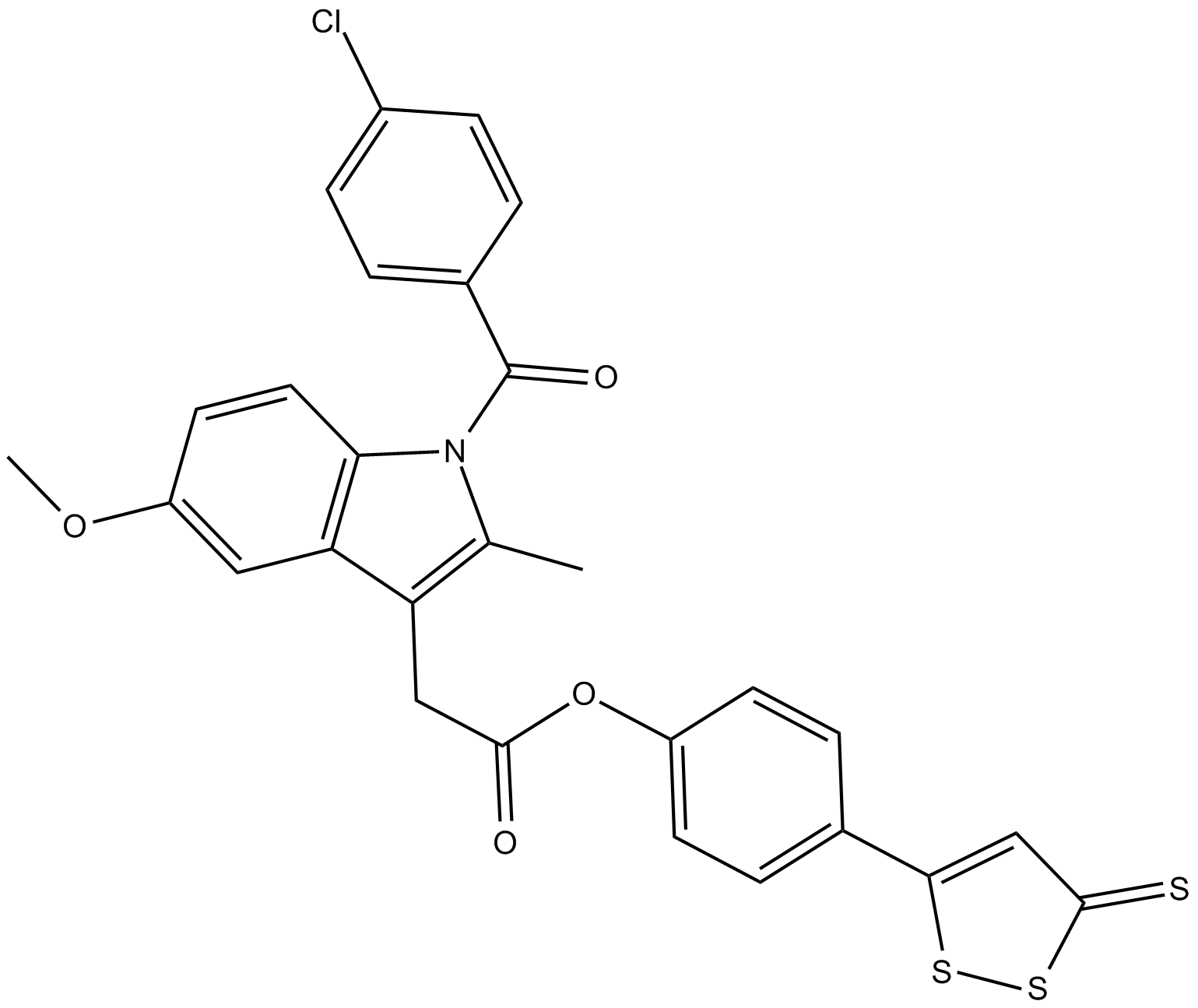
| Chemical Name | 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl ester-1H-indole-3-acetic acid |
| SDF | Download SDF |
| Canonical SMILES | COc1ccc2c(c1)c(CC(=O)Oc1ccc(cc1)c1ssc(=S)c1)c(C)n2C(=O)c1ccc(Cl)cc1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure