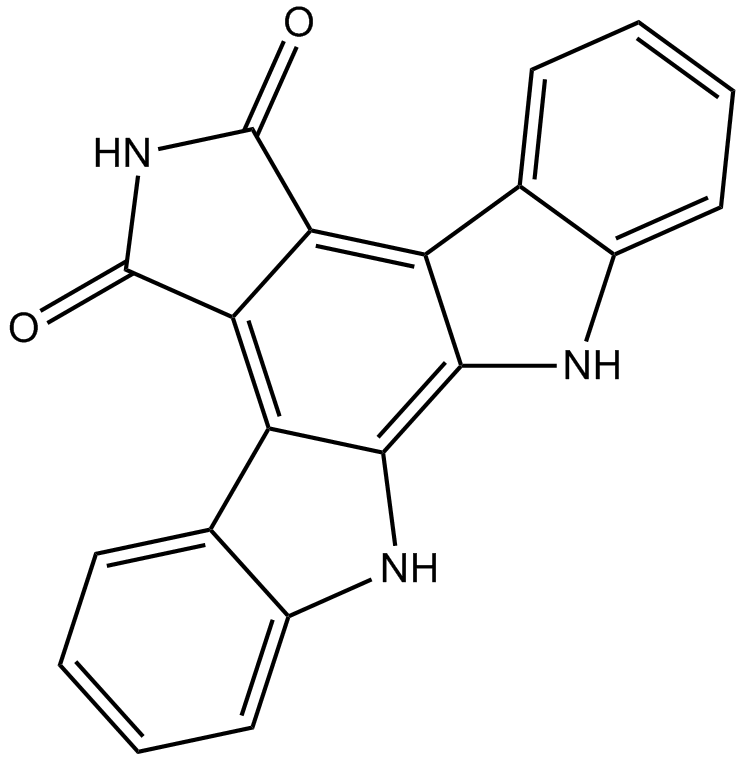
Arcyriaflavin A
IC50: 0.2 μM for HCMV [1], 0.14 μM for D1–CDK4 [2]
The natural product Arcyriaflavin A, unsubstituted indolocarbazole, was a potent selective inhibitor of human cytomegalovirus (HCMV) replication. HCMV infection is typically unnoticed in healthy people, but can be life-threatening for the immunocompromised.
In vitro: Arcyriaflavin A is a potent, selective inhibitor of HCMV replication in cell culture, and the anti-HCMV activity appeared no relation to the inhibition of protein kinase C. The imide NH was identified to be essential for anti-HCMV activity [1]. Arcyriaflavin A also has been showed the inhibitory activity against D1/CDK4 with a IC50 of 59 nM. Based on X-ray co-crystal structure of staurosporine and the human CDK2, the acidic proton of the maleimide moiety and the carbonyl group play critical roles by acting as a hydrogen bond donor and acceptor in the ATP binding pocket of CDK2 [2].
In vivo: So far, no in vivo study has been conducted.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Slater MJ, Cockerill S, Baxter R, Bonser RW, Gohil K, Gowrie C, Robinson JE, Littler E, Parry N, Randall R, Snowden W. Indolocarbazoles: potent, selective inhibitors of human cytomegalovirus replication. Bioorg Med Chem. 1999 Jun;7(6):1067-74.
[2] Zhu G, Conner S, Zhou X, Shih C, Brooks HB, Considine E, Dempsey JA, Ogg C, Patel B, Schultz RM, Spencer CD, Teicher B, Watkins SA. Synthesis of quinolinyl/isoquinolinyl[a]pyrrolo [3,4-c] carbazoles as cyclin D1/CDK4 inhibitors. Bioorg Med Chem Lett. 2003 Apr 7;13(7):1231-5.
| Physical Appearance | Orange solid |
| Storage | Store at -20°C |
| M.Wt | 325.32 |
| Cas No. | 118458-54-1 |
| Formula | C20H11N3O2 |
| Solubility | ≥7.36 mg/mL in DMSO with ultrasonic; insoluble in EtOH; insoluble in H2O |
| Chemical Name | 12,13-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7(6H)-dione |
| SDF | Download SDF |
| Canonical SMILES | O=C(c(c1c2[nH]c3c1cccc3)c1c3c2[nH]c2c3cccc2)NC1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure