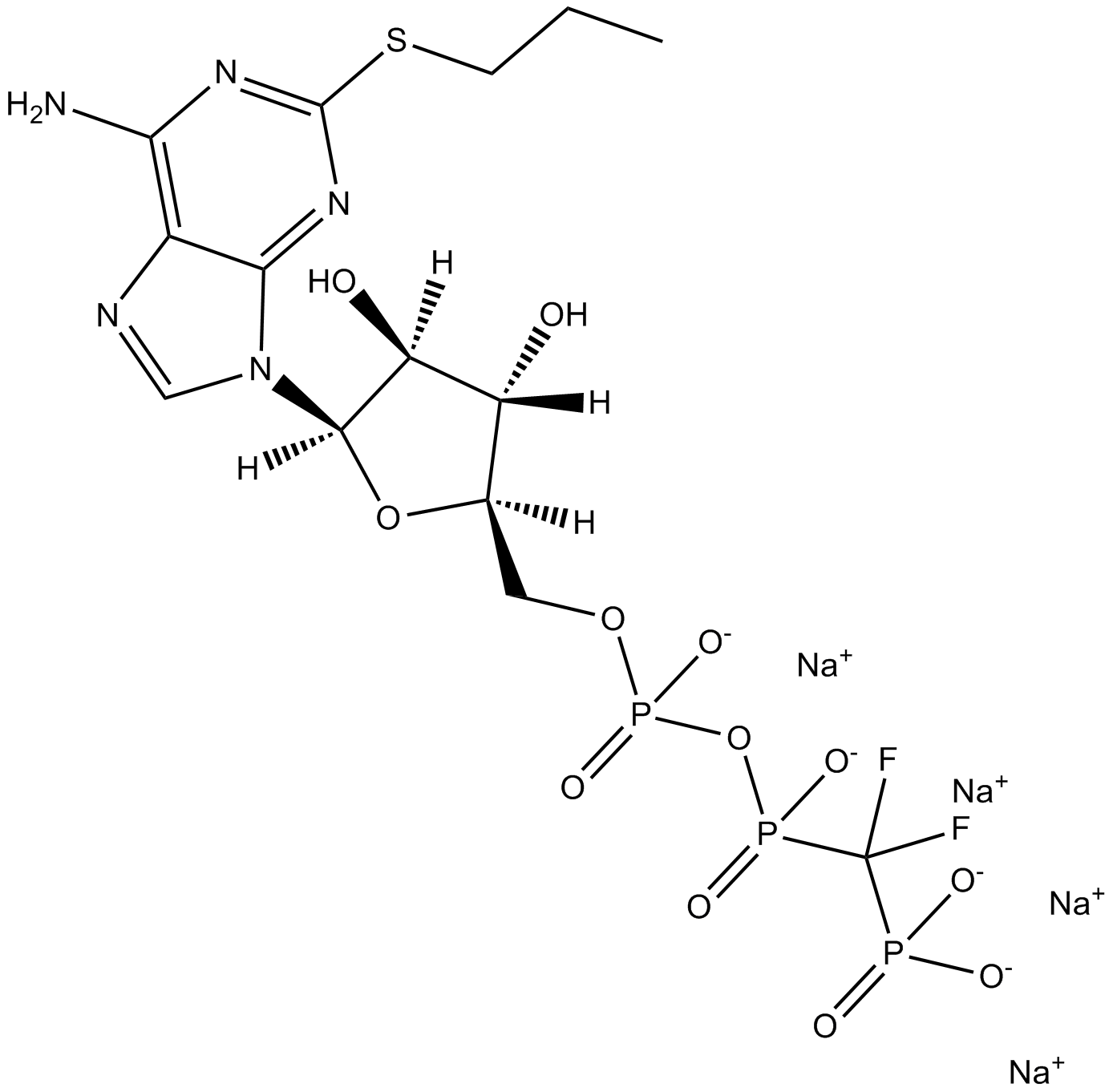
AR-C 66096 tetrasodium salt
The platelet P2T receptor plays a major role in platelet aggregation, and its antagonists are suggested to have significant therapeutic potential as antithrombotic agents. AR-C 66096 is a novel, potent and selective antagonist at human platelet P2T-purinoceptors.
In vitro: In suspensions of human washed platelets, AR-C 66096 (1-100 nM) produced concentrationdependent rightward displacement of concentration-effect (E/[A]) curves obtained for ADP-induced platelet aggregation. The anti-aggregatory potency of AR-C 66096 was not influenced by increasing the incubation time from 2 to 15 min nor by inclusion of the P1-purinoceptor antagonist 8-sulphophenyltheophylline at a concentration (300 μM) [1].
In vivo: AR-C 66096 behaved as a weak (pA50 3.68) but full P2x-purinoceptor agonist in preparations of the isolated rabbit ear artery and as a weak, competitive antagonist (apparent pKB 4.71) at P2Y-purinoceptors in the isolated guinea-pig aorta, indicating a selectivity of at least 9000 fold for the Pzrsubtype. In the latter preparation, non-specific relaxations were observed by concentrations of AR-C 66096 >10 μM [1].
Clinical trial: Up to now, AR-C 66096 is still in the preclinical development stage.
Reference:
[1] Humphries, R. G.; Tomlinson, W.; Ingall, A. H.; Cage, P. A.; Leff, P. FPL 66096: A Novel, Highly Potent and Selective Antagonist at Human Platelet P2T-purinoceptors. Br. J. Pharmacol. 1994, 113, 1057-1063.
| Physical Appearance | Colourless liquid |
| Storage | Store at -20°C |
| M.Wt | 703.26 |
| Cas No. | 145782-74-7 |
| Formula | C14H18F2N5Na4O12P3S |
| Solubility | <10mM in H2O |
| SDF | Download SDF |
| Canonical SMILES | CCCSC1=NC(N)=C(N=CN2[C@@]3([H])[C@](O)([H])[C@@](O)([H])[C@@](O3)([H])COP([O-])(OP(C(F)(P([O-])([O-])=O)F)([O-])=O)=O)C2=N1.[Na+].[Na+].[Na+].[Na+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure