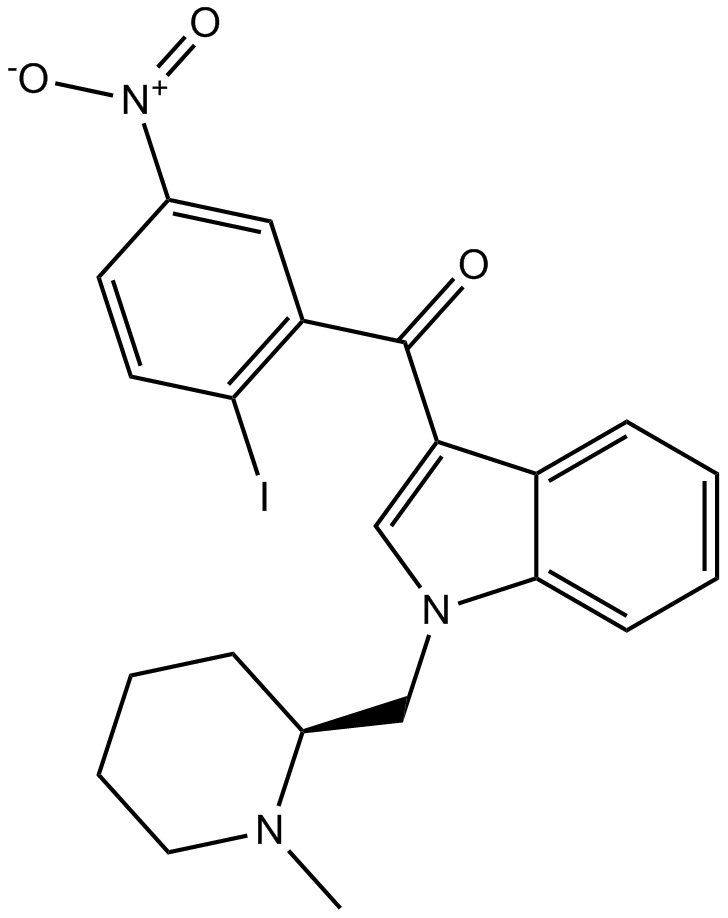
AM1241
AM1241 (CAS number: 444912-48-5) is a selective agonist of the cannabinoid receptor type 2 (CB2), a G protein-coupled receptor encoded by the CNR2 gene. CB2 receptors modulate inflammation and neuropathic pain pathways outside the central nervous system. In vitro studies suggest stereoisomer-dependent activities; specifically, the S-enantiomer demonstrates higher functional potency toward human and rodent CB2 receptors compared to the R-enantiomer. In animal models, AM1241 administration reduces neuropathic pain phenotypes including hyperalgesia and allodynia and shows potential for delaying disease progression in an ALS (amyotrophic lateral sclerosis) mouse model. Currently, AM1241 remains a valuable investigational tool in preclinical pain and neurodegeneration research.
- 1. Di Cui, Chuhua Yang, et al. "CB2 receptor agonist AM1241 regulating the polarization of microglia reduces morphine tolerance through IL-4/STAT6 pathway." Mol Pain. 2025 Aug 18:17448069251374281. PMID: 40823889
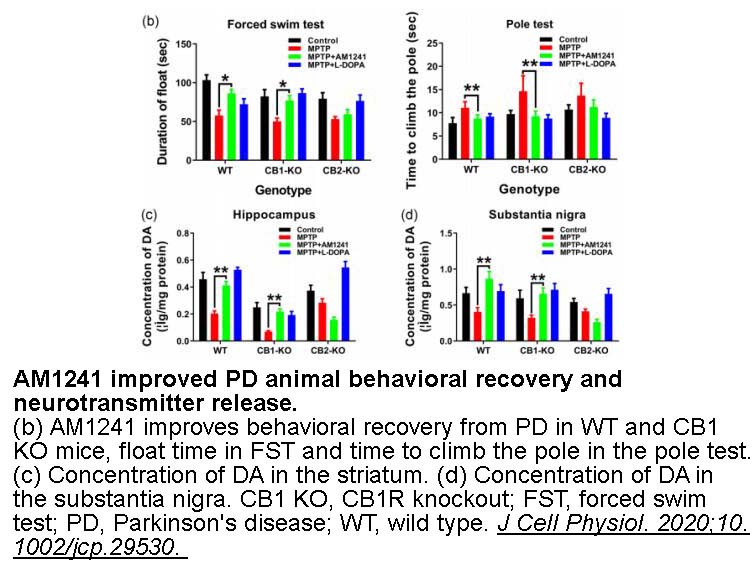
- 2. He X, Yang L, et al. "Activation of CB2R with AM1241 ameliorates neurodegeneration via the Xist/miR-133b-3p/Pitx3 axis." J Cell Physiol. 2020;10.1002/jcp.29530. PMID: 31989652
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 503.33 |
| Cas No. | 444912-48-5 |
| Formula | C22H22IN3O3 |
| Solubility | ≥50.3 mg/mL in DMSO with gentle warming; insoluble in H2O; ≥3.87 mg/mL in EtOH with ultrasonic |
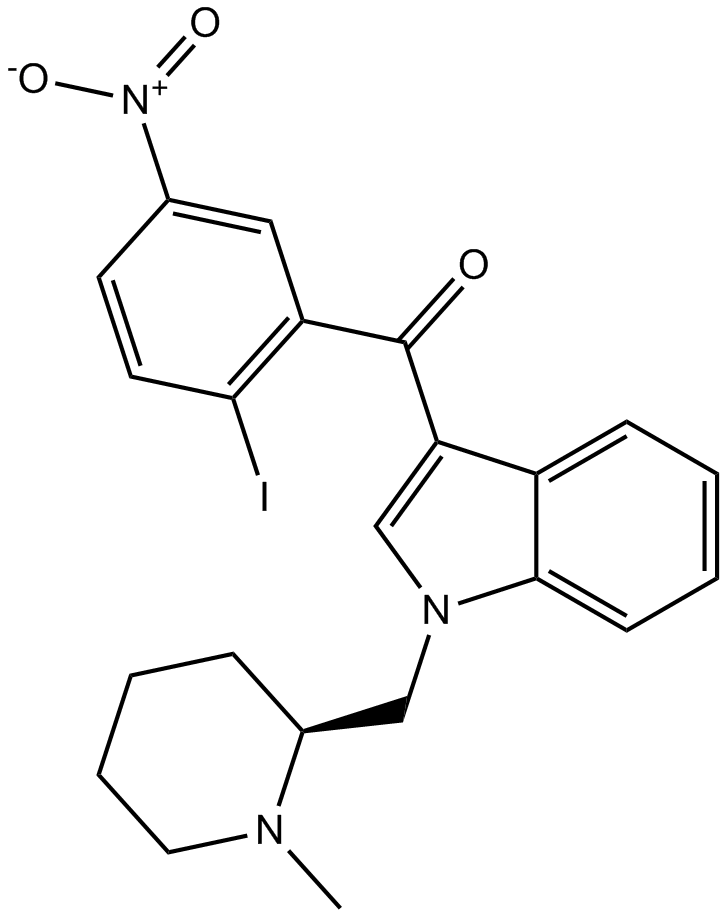
| Chemical Name | (2-iodo-5-nitrophenyl)-[1-[(1-methylpiperidin-2-yl)methyl]indol-3-yl]methanone |
| SDF | Download SDF |
| Canonical SMILES | CN1CCCCC1CN2C=C(C3=CC=CC=C32)C(=O)C4=C(C=CC(=C4)[N+](=O)[O-])I |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Binding assays |
Membrane samples were prepared from HEK cells stably expressing the human CB2 receptors previously generated, or the CHO cell line that stably expresses the human CB1 receptor. Briefly, the cells were harvested and homogenized using a Polytron for 2 × 10s bursts in a buffer containing 50 mM Tris-HCl, pH 7.4, 1 mM MgCl2, and 1 mM EDTA in the presence of protease inhibitors followed by centrifugation at 45000g for 20min. The membrane pellets were washed and frozen at -80°C in aliquots until use. Saturation binding reactions were performed at 30°C for 90min using [3H]CP 55,940 (0.01–8nm) in an assay buffer containing 50mm Tris-HCl, pH 7.4, 2.5mM EDTA, 5mM MgCl2, and 0.05% fatty acid free bovine serum albumin (BSA) and the reactions were terminated by rapid vacuum filtration through UniFilter-96 GF/C filter plates and four washes with cold assay buffer. Competition experiments were conducted using 0.5nM [3H]CP 55,940 in the presence of test compounds (0.1nM–10μM). |
| Cell experiment [1]: | |
|
Cell lines |
Human embryonic kidney (HEK) cells stably expressing the human CB2 receptor, Chinese hamster ovary (CHO) cell line stably expressing the human CB1 receptor |
|
Preparation method |
The solubility of this compound in DMSO is > 25.2 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
Ki:~7 nM (human CB2 receptor) |
|
Applications |
In HEK cells stably expressing the human CB2 receptor, AM1241 exhibited antagonist activity, blocking the agonist CP 55,940-evoked Ca2+ response in a concentration dependent manner with a Kb value of 63nM. In [3H]CP 55,940 competition binding assays, AM-1241 displayed high affinity at the human CB2 receptor with a Ki value of ~7 nM, whereas its affinity at the human CB1 receptor was more than 80-fold weaker, using membrane preparations from stable HEK and CHO cell lines expressing the recombinant human CB2 and CB1 receptors, respectively. |
| Animal experiment [2]: | |
|
Animal models |
Adult male Sprague–Dawley rats |
|
Dosage form |
Intraperitoneal injection, 100, 330 μg/kg |
|
Application |
AM1241 (100, 330 μg/kg i.p.) suppressed the development of carrageenan-evoked thermal and mechanical hyperalgesia and allodynia. Intraplantar (ipl) administration of AM1241 (33 μg/kg ipl) suppressed hyperalgesia and allodynia following administration to the carrageenan-injected paw but was inactive following administration in the contralateral (noninflamed) paw. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Yao B B, Mukherjee S, Fan Y, et al. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor[J]. British journal of pharmacology, 2006, 149(2): 145-154. [2]. Nackley A G, Makriyannis A, Hohmann A G. Selective activation of cannabinoid CB 2 receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation[J]. Neuroscience, 2003, 119(3): 747-757. |
|
| Description | AM-1241 is a selective agonist of cannabinoid CB2 receptor with a Ki value of 3.4 nM. | |||||
| Targets | CB2 | CB1 | ||||
| IC50 | 3.4 nM(Ki) | 280 nM(Ki) | ||||
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure
Related Biological Data