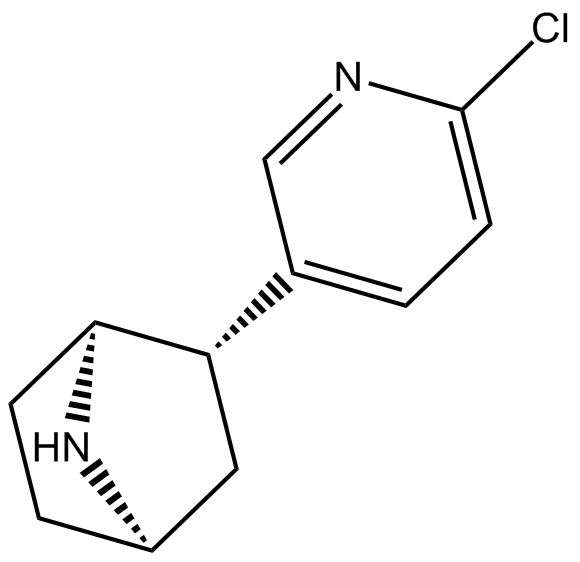
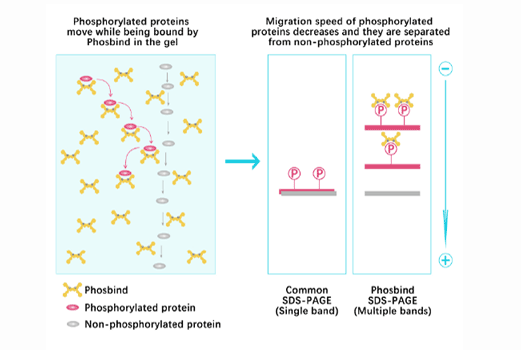
(±)-Epibatidine
(+)-AJ 76 hydrochloride is an antagonist of dopamine autoreceptor with pKi values of 6.95, 6.67, 6.37, 6.21 and 6.07 for hD3, hD4, hD2S, hD2L and rD2 receptors, respectively.
Dopamine receptor is a G protein-coupled receptor and mainly exists in the vertebrate central nervous system (CNS). Dopamine receptor is a receptor for dopamine and plays a critical role in memory, learning, pleasure, cognition, motivation and fine motor control.
(+)-AJ 76 hydrochloride is a dopamine receptor antagonist. In rats, AJ76 stimulated locomotor activity and increased the levels of 3,4-dihydroxyphenylacetic acid (DOPAC) and HVA in brain, which were dopamine metabolites [1]. In rats injected with cocaine, (+)-A J76 increased the locomotor stimulation during the first 30 min. However, (+)-AJ76 inhibited the later more intense locomotor stimulation and cocaine-induced stereotypies [2]. In vivo, (+)-AJ76 induced dopamine release mainly through interaction with dopamine receptors in the terminal regions of the A9 and A10 dopaminergic fibers. However, (+)-AJ76 increased the level of DOPAC via the somatodendritic autoreceptors [3].
References:
[1]. Kullingsjö H, Carlsson A, Svensson K. Effects of repeated administration of the preferential dopamine autoreceptor antagonist, (+)-AJ76, on locomotor activity and brain DA metabolism in the rat. Eur J Pharmacol, 1991, 205(3): 241-246.
[2]. Piercey MF, Lum JT, Hoffmann WE, et al. Antagonism of cocaine's pharmacological effects by the stimulant dopaminergic antagonists, (+)-AJ76 and (+)-UH232. Brain Res, 1992; 588(2): 217-222.
[3]. Waters N, Hansson L, Löfberg L, et al. Intracerebral infusion of (+)-AJ76 and (+)-UH232: effects on dopamine release and metabolism in vivo. Eur J Pharmacol, 1994, 251(2-3): 181-190.
| Physical Appearance | Off White solid |
| Storage | Store at -20°C |
| M.Wt | 208.69 |
| Cas No. | 148152-66-3 |
| Formula | C11H13ClN2 |
| Solubility | insoluble in H2O; <24.55mg/ml in ethanol |
| Chemical Name | (1S,2S,4R)-2-(6-chloropyridin-3-yl)-7-azabicyclo[2.2.1]heptane |
| SDF | Download SDF |
| Canonical SMILES | Clc1ncc([C@H]2[C@H](CC3)N[C@H]3C2)cc1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure