Search results for: 'research area proteases heat shock proteins hsp'
-
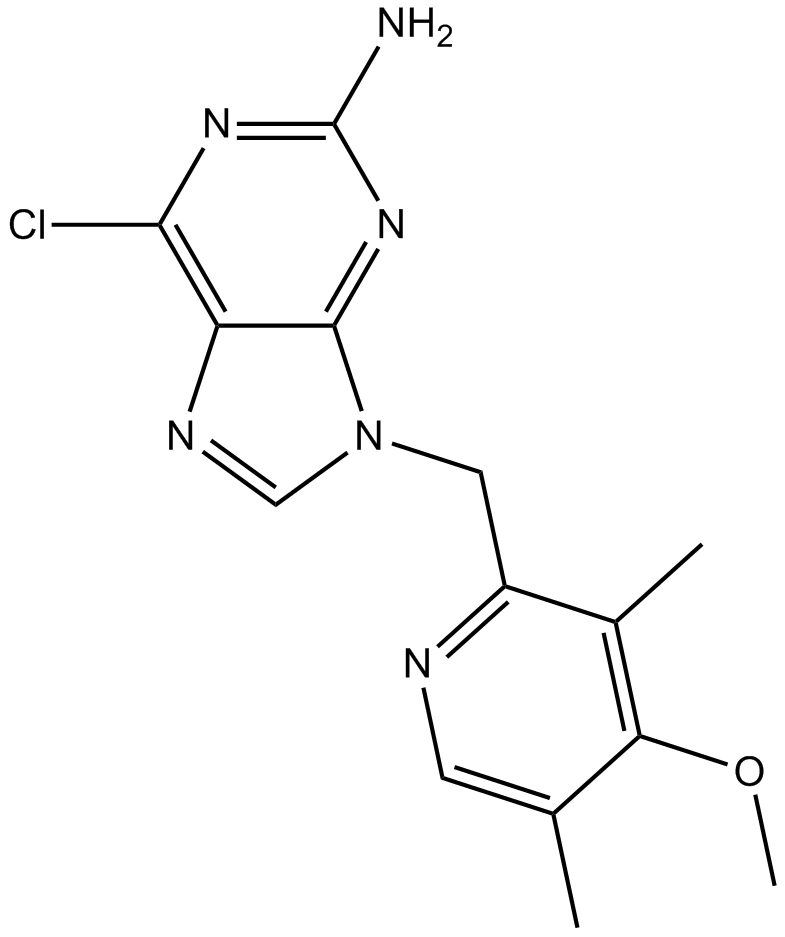 A4058 BIIB021Target: HSP90Summary: Hsp90 inhibitor,selective and competitive
A4058 BIIB021Target: HSP90Summary: Hsp90 inhibitor,selective and competitive -
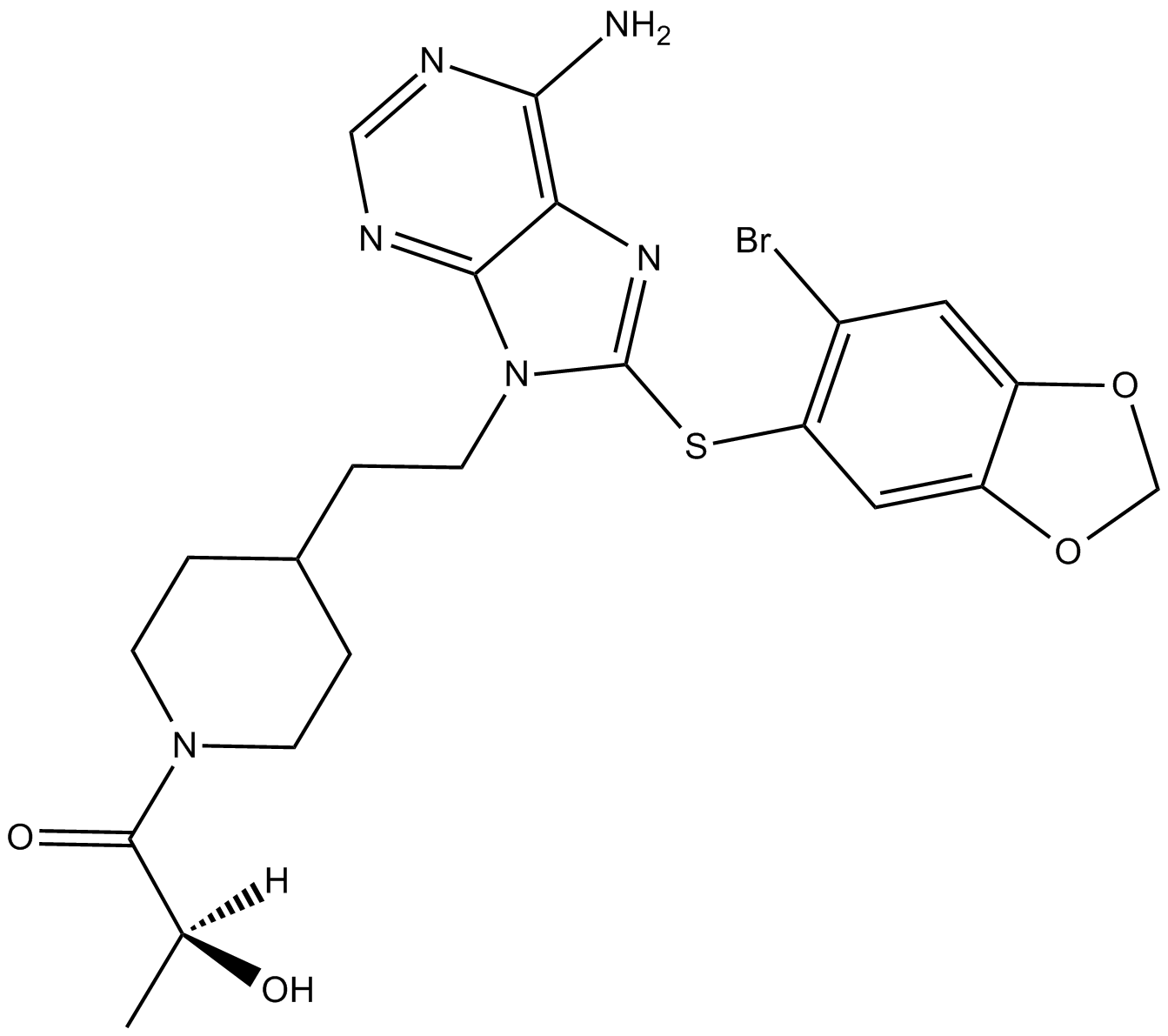 A4063 MPC-3100Summary: Hsp90 inhibitor
A4063 MPC-3100Summary: Hsp90 inhibitor -
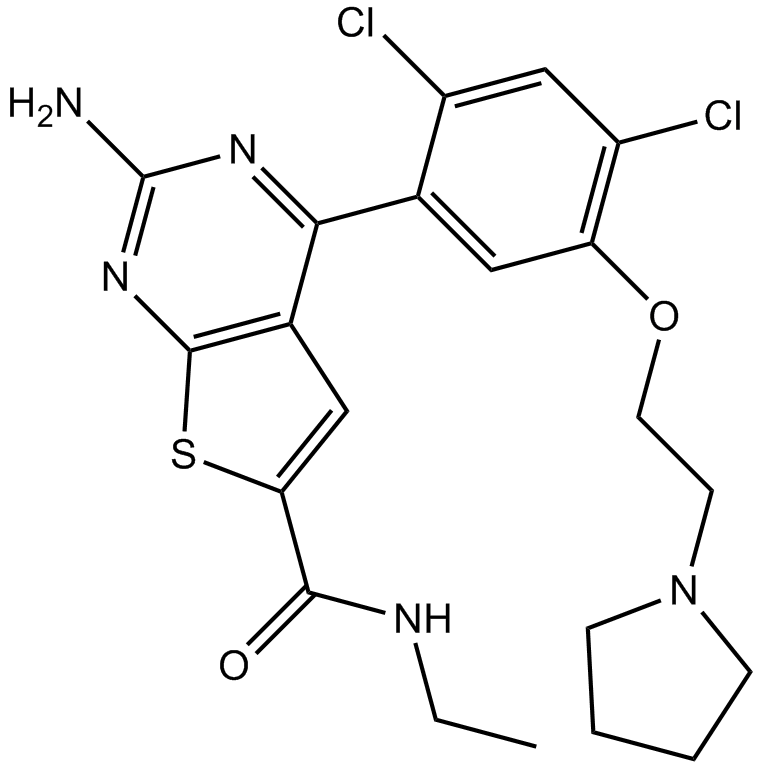 A4064 NVP-BEP800Target: HSP90Summary: Oral Hsp90β inhibitor, novel, fully synthetic
A4064 NVP-BEP800Target: HSP90Summary: Oral Hsp90β inhibitor, novel, fully synthetic -
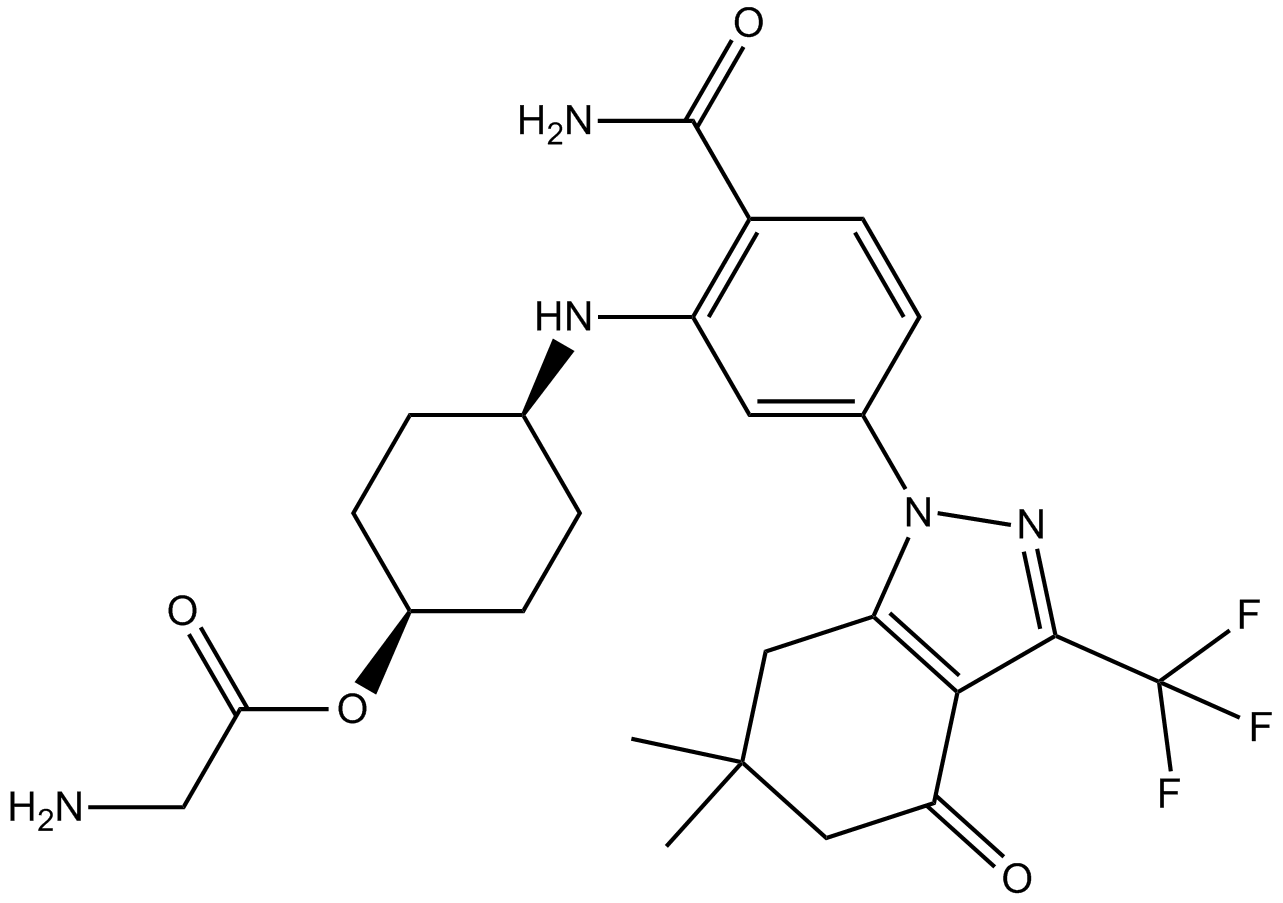 A4065 PF-04929113 (SNX-5422)Target: HSP90Summary: Hsp90 inhibitor,potent and selective
A4065 PF-04929113 (SNX-5422)Target: HSP90Summary: Hsp90 inhibitor,potent and selective -
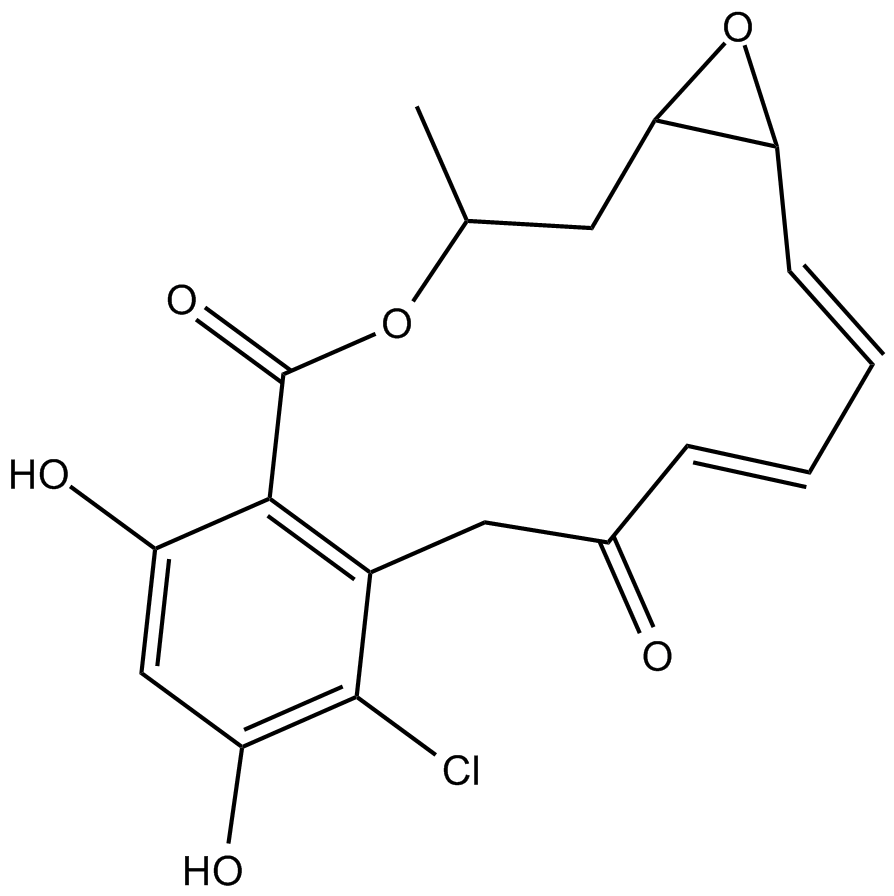 A4067 RadicicolSummary: ATPase/kinase inhibitor
A4067 RadicicolSummary: ATPase/kinase inhibitor -
 P1070 Heat Shock Protein 90 (Hsp90), human recombinant proteinSummary: Heat Shock Protein 90, expressed in Sf9 cells, suitable for cell culture.
P1070 Heat Shock Protein 90 (Hsp90), human recombinant proteinSummary: Heat Shock Protein 90, expressed in Sf9 cells, suitable for cell culture. -
 P1071 Heat Shock Protein 70, human recombinant proteinSummary: Heat Shock Protein 70, expressed in E. coli, suitable for cell culture.
P1071 Heat Shock Protein 70, human recombinant proteinSummary: Heat Shock Protein 70, expressed in E. coli, suitable for cell culture. -
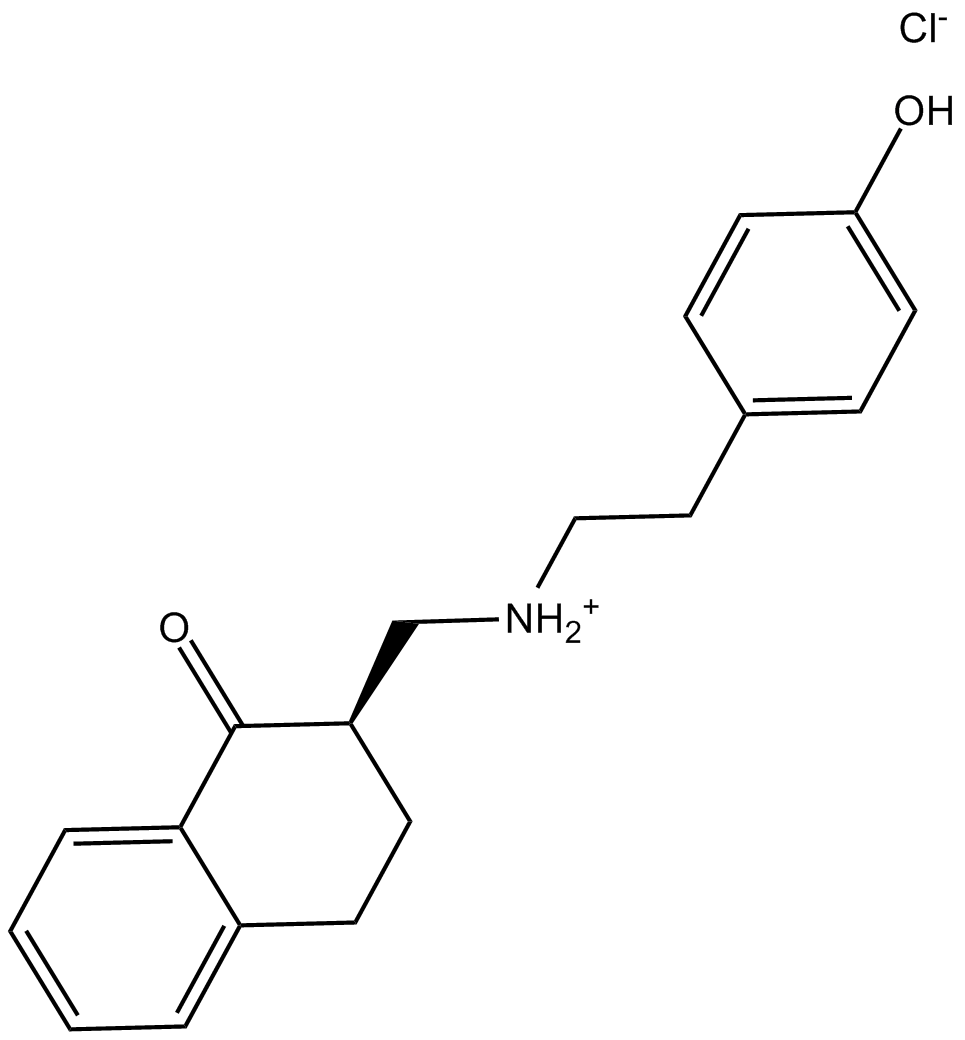 B6339 HEAT hydrochlorideSummary: α1-adrenoceptor antagonist
B6339 HEAT hydrochlorideSummary: α1-adrenoceptor antagonist -
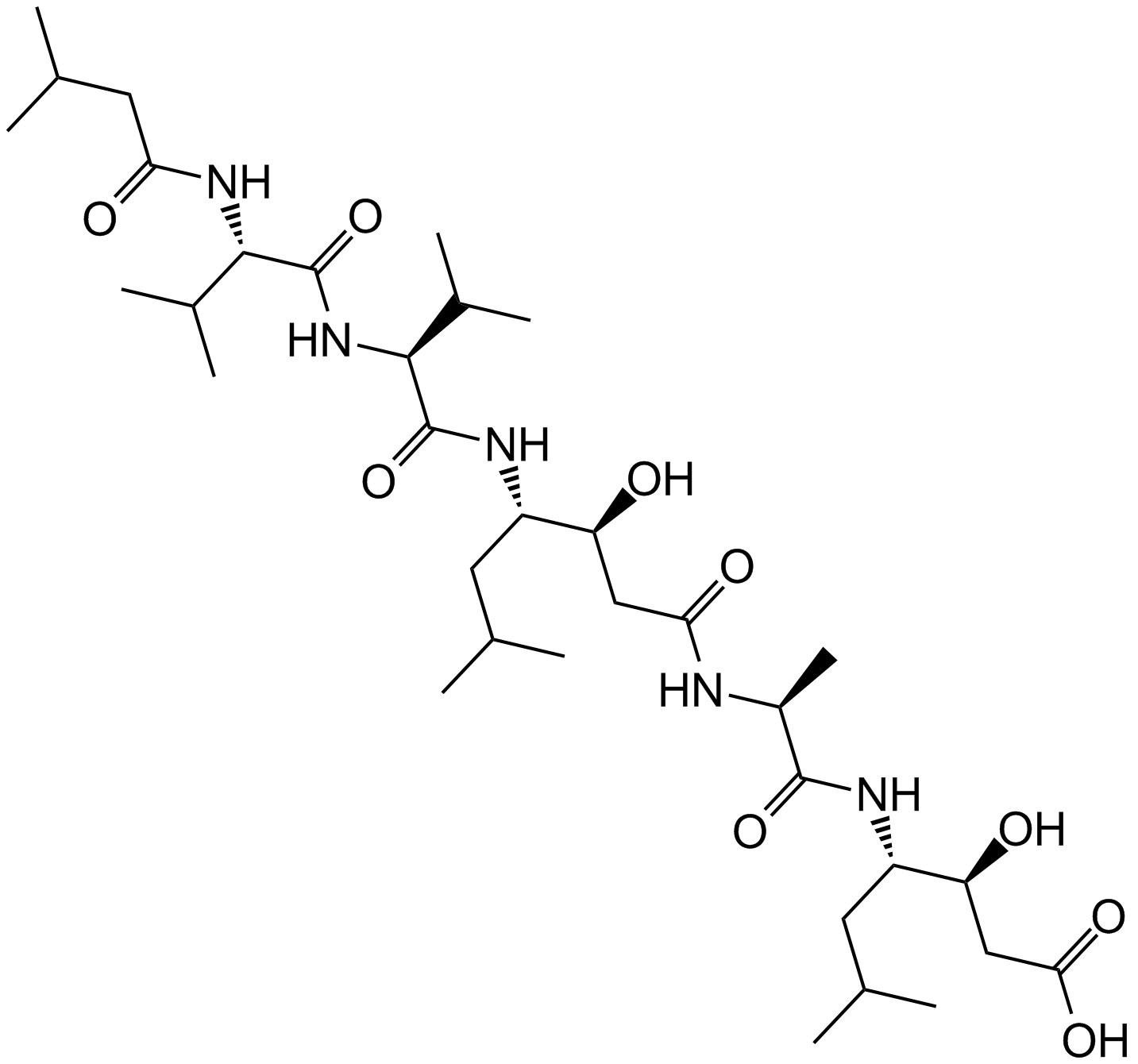 A2571 Pepstatin A9 CitationTarget: Cathepsins|Renin|HIV proteases|PepsinsSummary: aspartic proteases inhibitor
A2571 Pepstatin A9 CitationTarget: Cathepsins|Renin|HIV proteases|PepsinsSummary: aspartic proteases inhibitor -
 A4446 R18Summary: 14.3.3 proteins Antagonist
A4446 R18Summary: 14.3.3 proteins Antagonist


