Search results for: 'research area apoptosis'
-
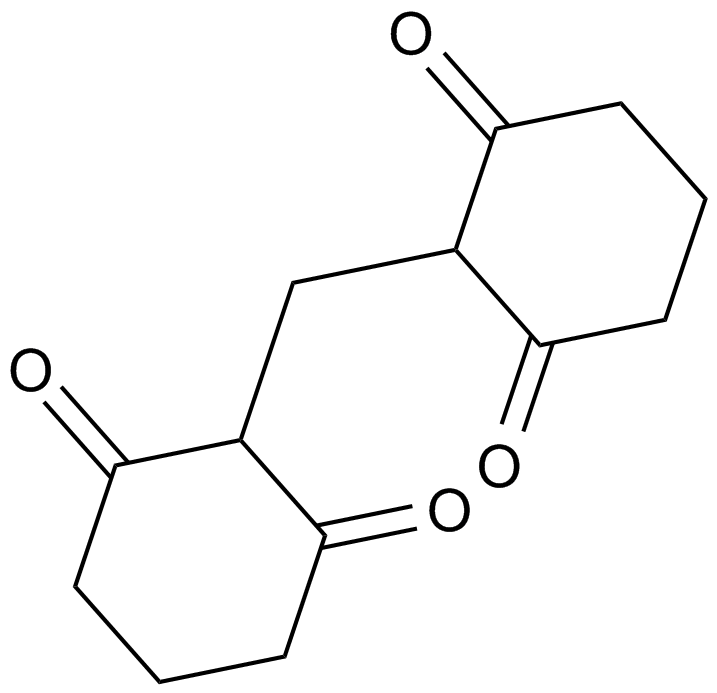 A1930 Apoptosis InhibitorSummary: Associate with caspase-3 inhibition
A1930 Apoptosis InhibitorSummary: Associate with caspase-3 inhibition -
 K2711 Apoptosis Inducer KitSummary: A ready-to-use apoptosis inducer reagent, which consists of recombinant human TNF-α and SM-164
K2711 Apoptosis Inducer KitSummary: A ready-to-use apoptosis inducer reagent, which consists of recombinant human TNF-α and SM-164 -
 A4016 Apoptosis Activator 2Summary: Indoledione caspase activator, cell-permeable
A4016 Apoptosis Activator 2Summary: Indoledione caspase activator, cell-permeable -
 L1036 DiscoveryProbe™ Apoptosis Compound LibrarySummary: A unique collection of 643 apoptosis-related compounds for apoptosis research.
L1036 DiscoveryProbe™ Apoptosis Compound LibrarySummary: A unique collection of 643 apoptosis-related compounds for apoptosis research. -
 K2718 Apoptosis Inducer Set (Ready-to-use)Summary: A set of apoptosis inducers
K2718 Apoptosis Inducer Set (Ready-to-use)Summary: A set of apoptosis inducers -
 K2009 Annexin V-Biotin/PI Apoptosis KitSummary: Detects Apoptosis by FACS or FL.
K2009 Annexin V-Biotin/PI Apoptosis KitSummary: Detects Apoptosis by FACS or FL. -
 K2282 Annexin V-PE/DAPI Apoptosis KitSummary: Annexin V-PE/DAPI apoptosis detection kit, which can detect apoptosis& necrosis within 15-30 minutes
K2282 Annexin V-PE/DAPI Apoptosis KitSummary: Annexin V-PE/DAPI apoptosis detection kit, which can detect apoptosis& necrosis within 15-30 minutes -
 K2283 Annexin V-PE/7-AAD Apoptosis KitSummary: Annexin V-PE/7-AAD apoptosis detection kit, which can detect apoptosis& necrosis within 15-30 minutes
K2283 Annexin V-PE/7-AAD Apoptosis KitSummary: Annexin V-PE/7-AAD apoptosis detection kit, which can detect apoptosis& necrosis within 15-30 minutes -
 K2252 Annexin V-HF488/DAPI Apoptosis KitSummary: Annexin V-HF488/DAPI apoptosis detection kit, which can detect apoptosis& necrosis within 10-20 minutes
K2252 Annexin V-HF488/DAPI Apoptosis KitSummary: Annexin V-HF488/DAPI apoptosis detection kit, which can detect apoptosis& necrosis within 10-20 minutes -
 K2253 Annexin V-HF488/PI Apoptosis KitSummary: Annexin V-HF488/PI apoptosis detection kit, which can detect apoptosis& necrosis within 10-20 minutes
K2253 Annexin V-HF488/PI Apoptosis KitSummary: Annexin V-HF488/PI apoptosis detection kit, which can detect apoptosis& necrosis within 10-20 minutes

