Search results for: 'rapamycin'
-
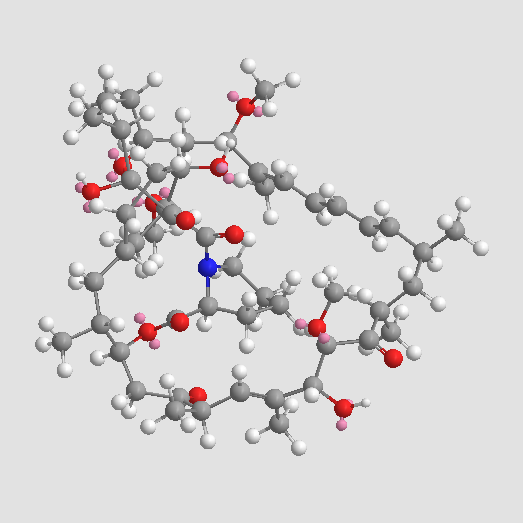 A8167 Rapamycin (Sirolimus)73 CitationTarget: mTORSummary: mTOR inhibitor
A8167 Rapamycin (Sirolimus)73 CitationTarget: mTORSummary: mTOR inhibitor -
 L1043 DiscoveryProbe™ MAPK Inhibitor LibrarySummary: A unique collection of 117 MAPK inhibitors for MAPK signaling pathway research.
L1043 DiscoveryProbe™ MAPK Inhibitor LibrarySummary: A unique collection of 117 MAPK inhibitors for MAPK signaling pathway research. -
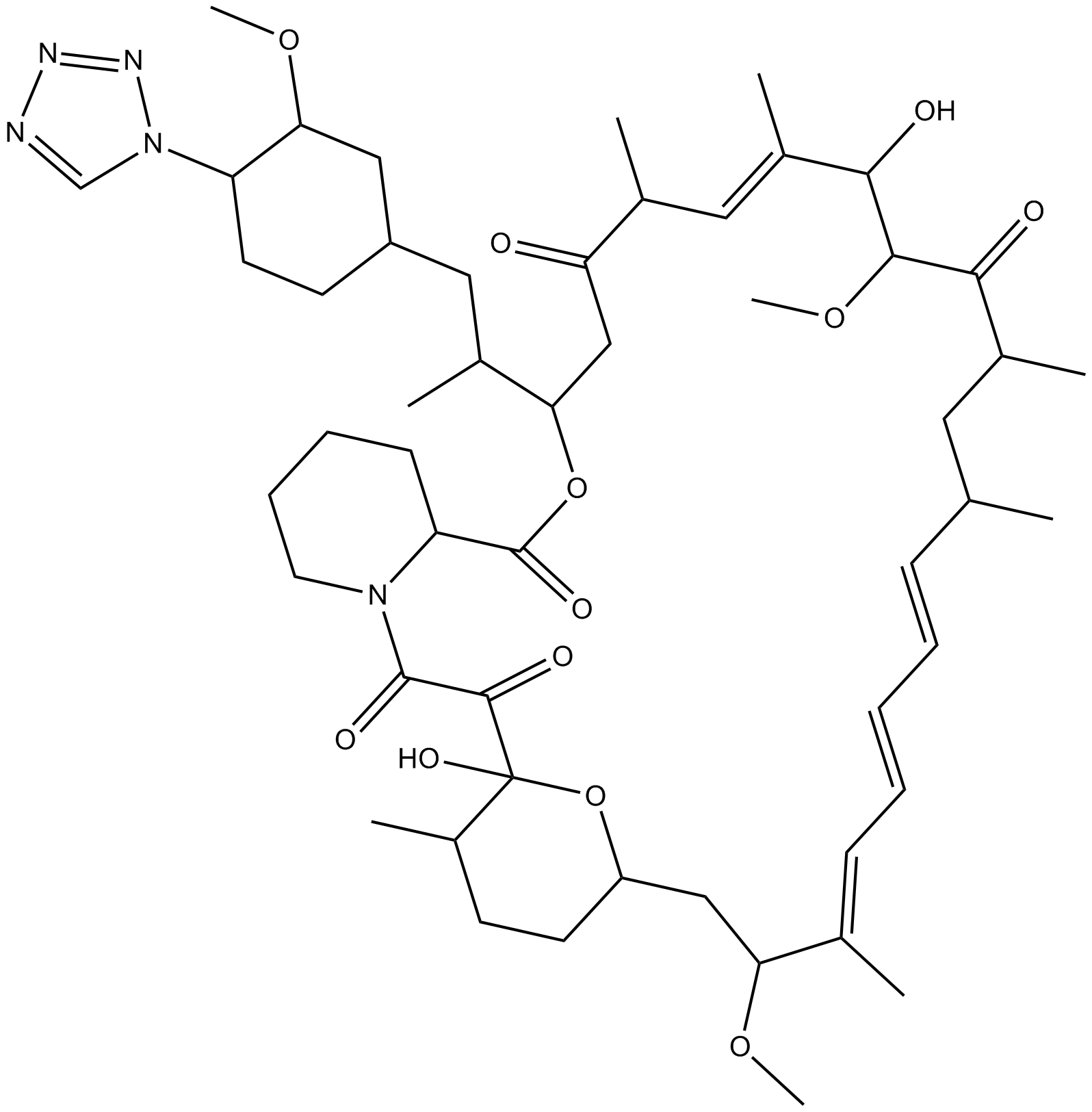 B4786 Zotarolimus(ABT-578)Summary: anti-proliferative drug used exclusively in coronary drug eluting stent
B4786 Zotarolimus(ABT-578)Summary: anti-proliferative drug used exclusively in coronary drug eluting stent -
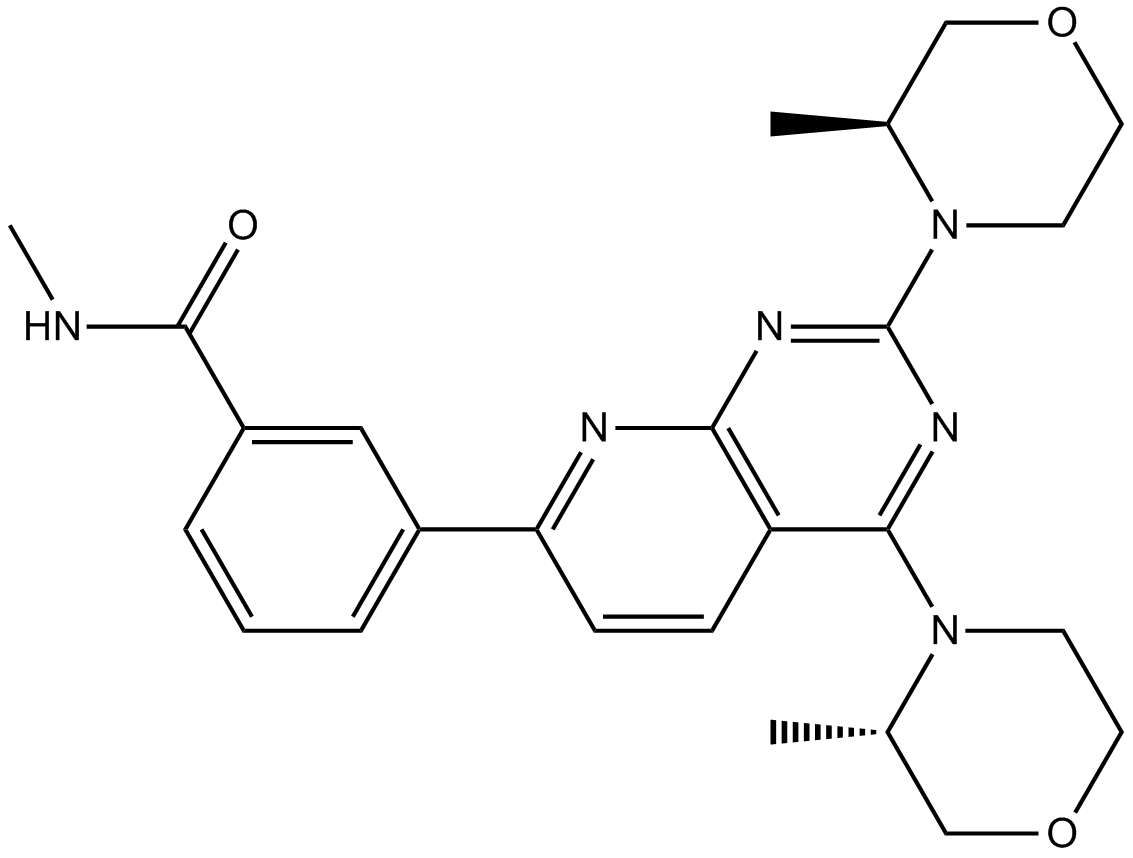 A8373 AZD2014Summary: Novel mTOR inhibitor
A8373 AZD2014Summary: Novel mTOR inhibitor -
 BA3662 SMER18Summary: A small molecule enhancer of Rapamycin that acts as an mTOR-independent inducer of autophagy.
BA3662 SMER18Summary: A small molecule enhancer of Rapamycin that acts as an mTOR-independent inducer of autophagy. -
 BA1298 UmirolimusSummary: Umirolimus is a derivative of the macrocyclic trienolide Rapamycin, a powerful immunosuppressant and anti-inflammatory agent.
BA1298 UmirolimusSummary: Umirolimus is a derivative of the macrocyclic trienolide Rapamycin, a powerful immunosuppressant and anti-inflammatory agent. -
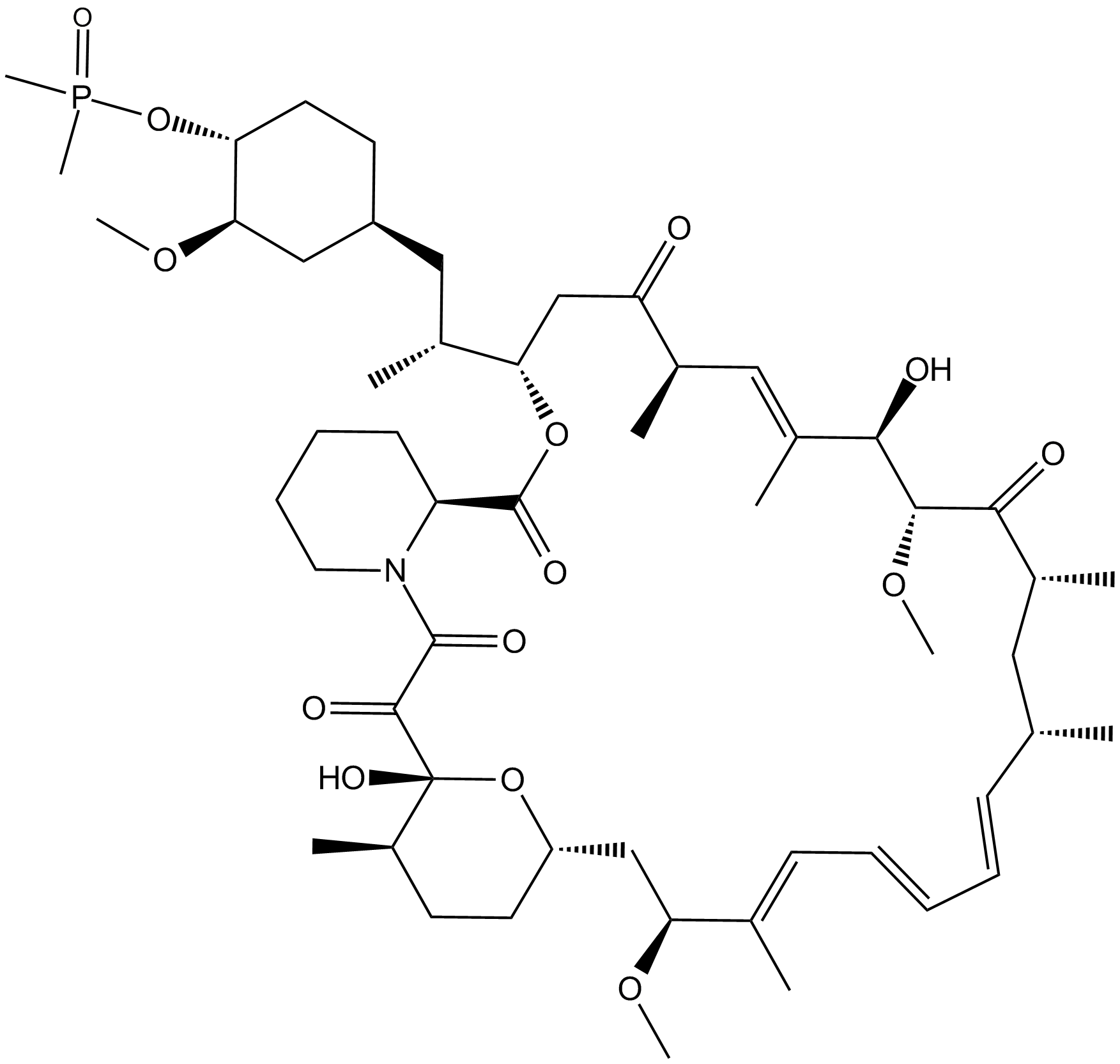 B1639 Ridaforolimus (Deforolimus, MK-8669)3 CitationTarget: mTORSummary: mTOR inhibitor
B1639 Ridaforolimus (Deforolimus, MK-8669)3 CitationTarget: mTORSummary: mTOR inhibitor -
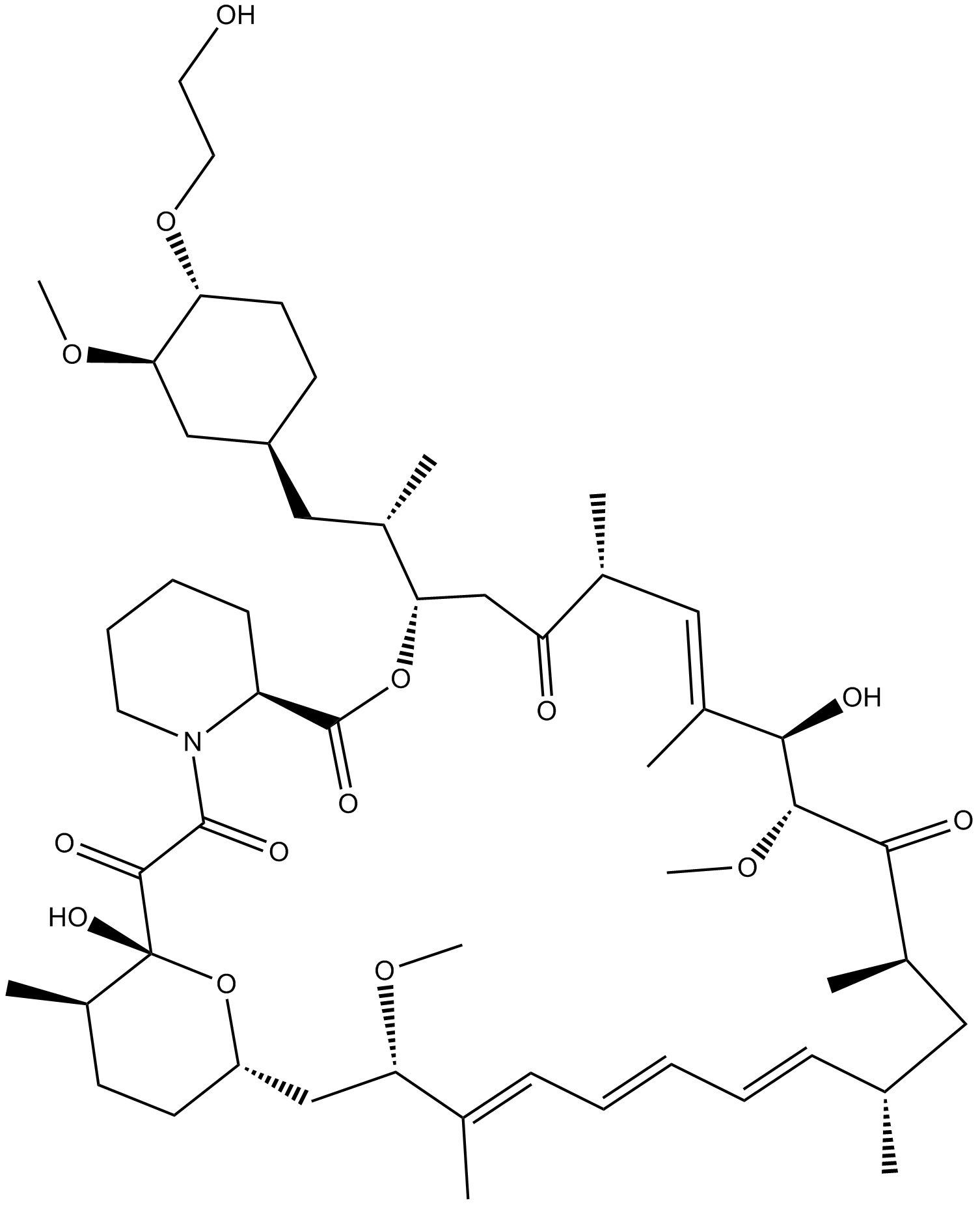 A8169 Everolimus (RAD001)17 CitationTarget: mTORSummary: MTOR inhibitor
A8169 Everolimus (RAD001)17 CitationTarget: mTORSummary: MTOR inhibitor -
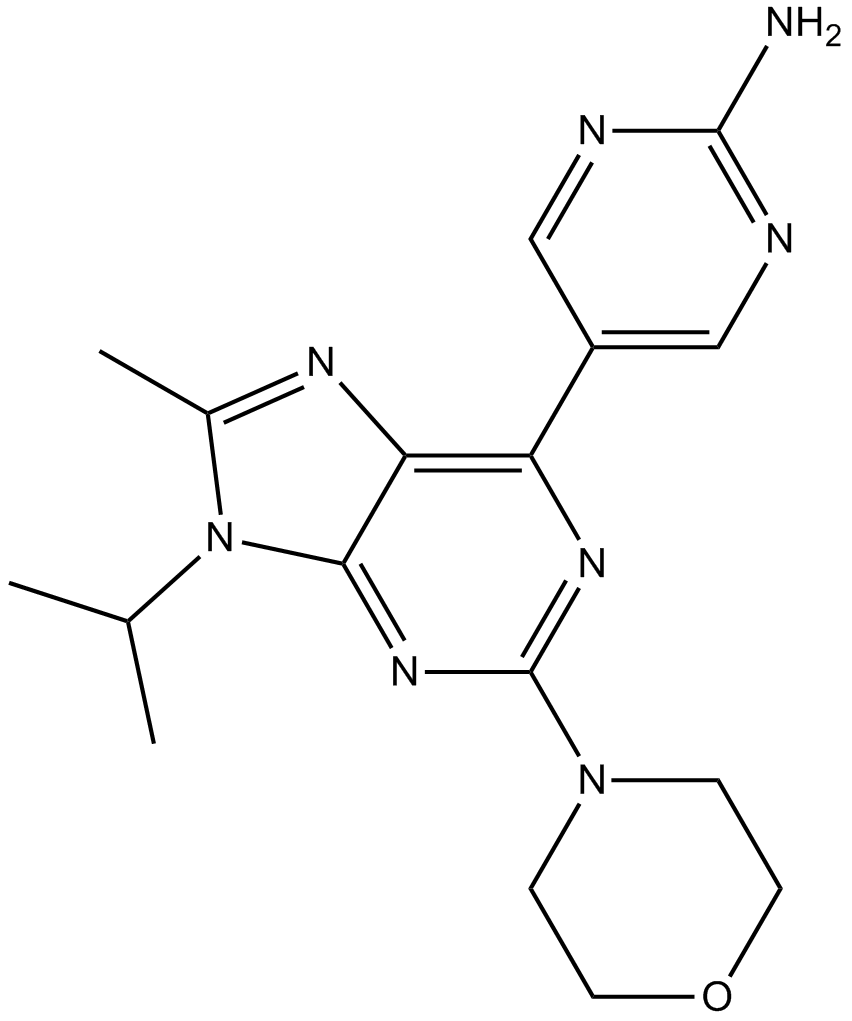 A3927 VS-5584 (SB2343)Target: PI3K|mTORSummary: MTOR/P13K inhibitor,potent and selective
A3927 VS-5584 (SB2343)Target: PI3K|mTORSummary: MTOR/P13K inhibitor,potent and selective


