Ubiquitination/ Proteasome
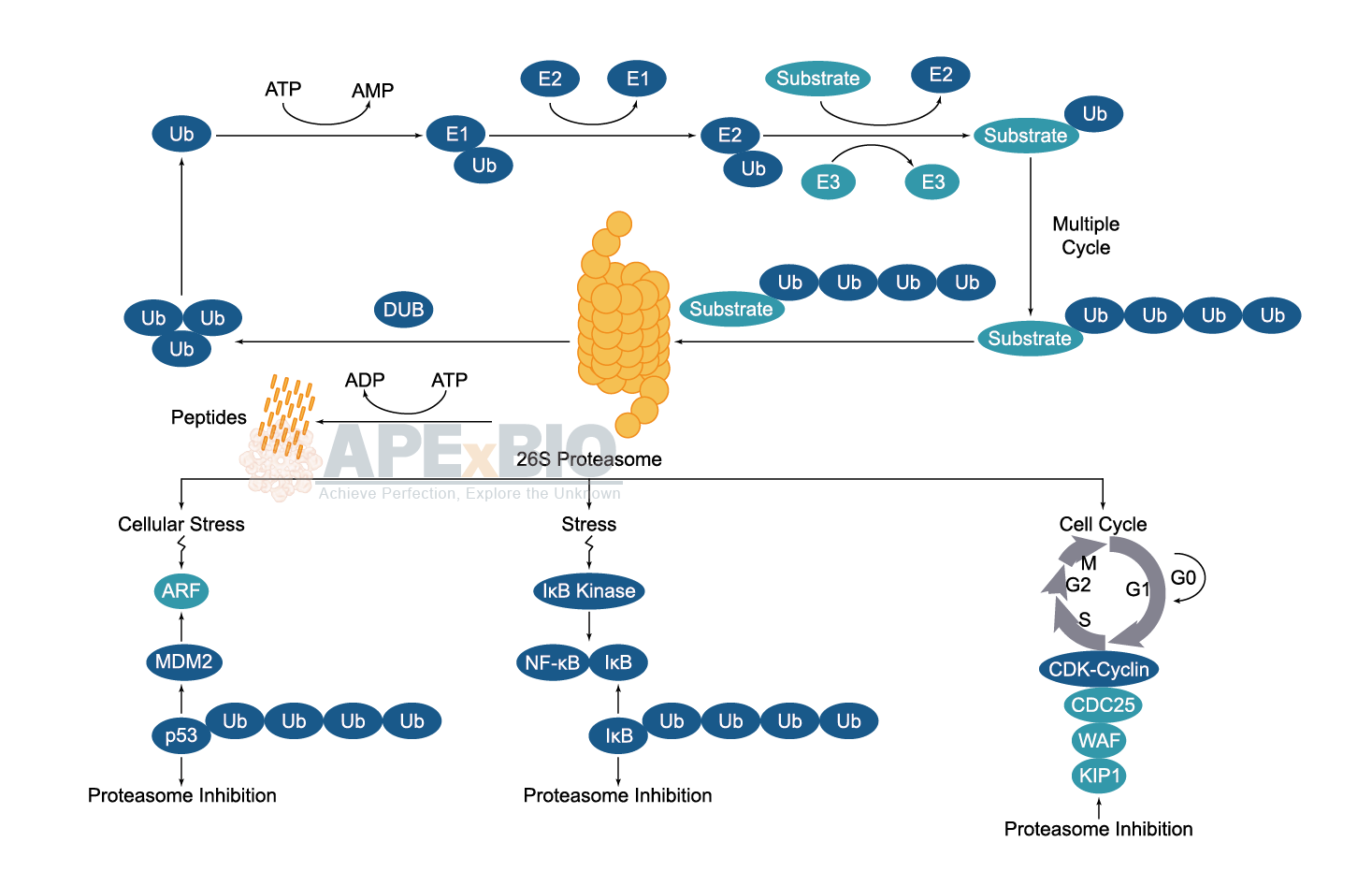
Once the substrate protein is labeled, proteasome will bind to a polyubiquitin chain, allowing the degradation of the labeled protein. The polyubiquitinated target protein is then recognized and degraded by the 26S proteasome. Deubiquitinating enzymes (DUBs) reverse the process of ubiquitination by removing ubiquitin from its substrate protein. Dysregulation of the ubiquitin-proteasome system has been linked to cancer, diabetes, cardiovascular and neurodegenerative diseases etc.
-
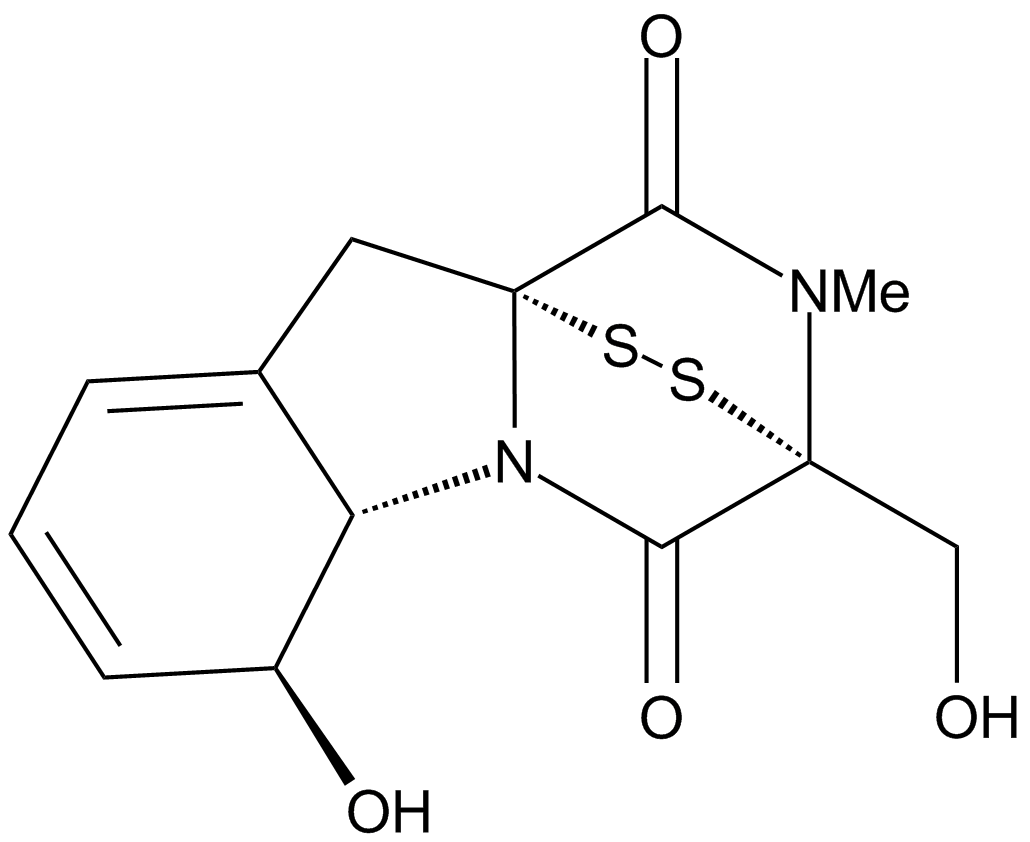 A4443 Gliotoxin1 CitationTarget: 20S proteasomal chymotrypsin|Geranylgeranyltransferase I|FarnesyltransferaseSummary: 20S proteasome inhibitor
A4443 Gliotoxin1 CitationTarget: 20S proteasomal chymotrypsin|Geranylgeranyltransferase I|FarnesyltransferaseSummary: 20S proteasome inhibitor -
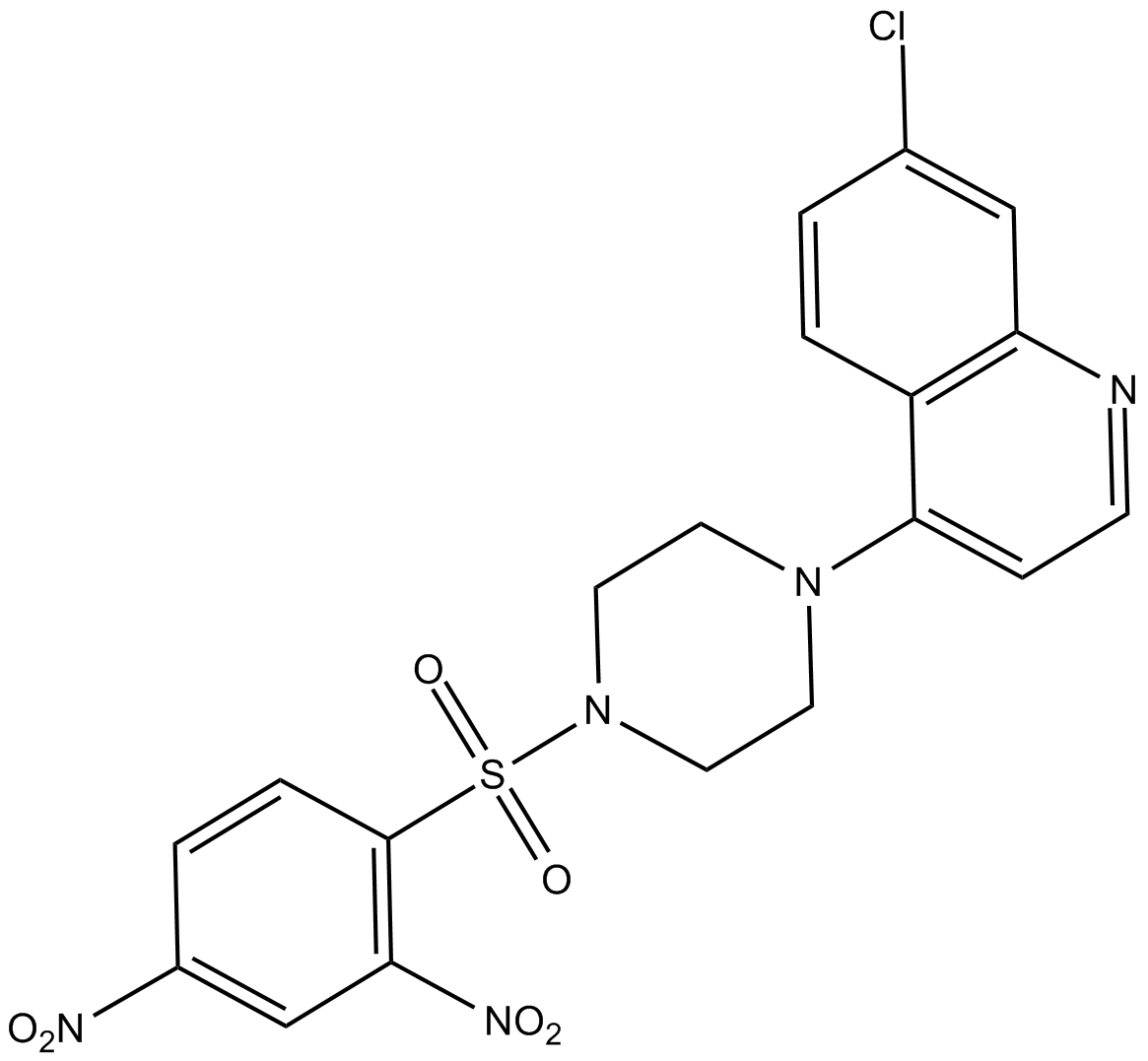 B6026 VR23Target: ProteasomeSummary: proteasome inhibitor
B6026 VR23Target: ProteasomeSummary: proteasome inhibitor -
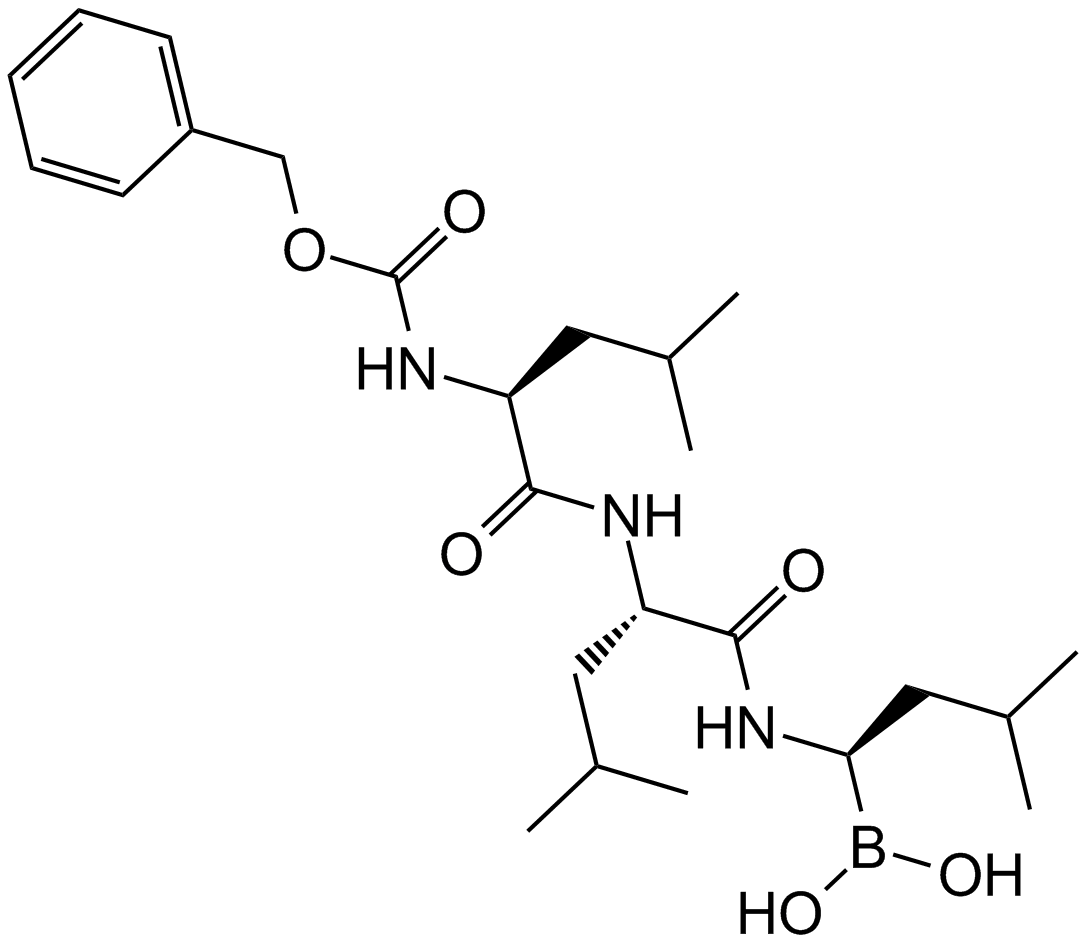 A8179 MG-2621 CitationTarget: ProteasomeSummary: Proteasome inhibitor
A8179 MG-2621 CitationTarget: ProteasomeSummary: Proteasome inhibitor -
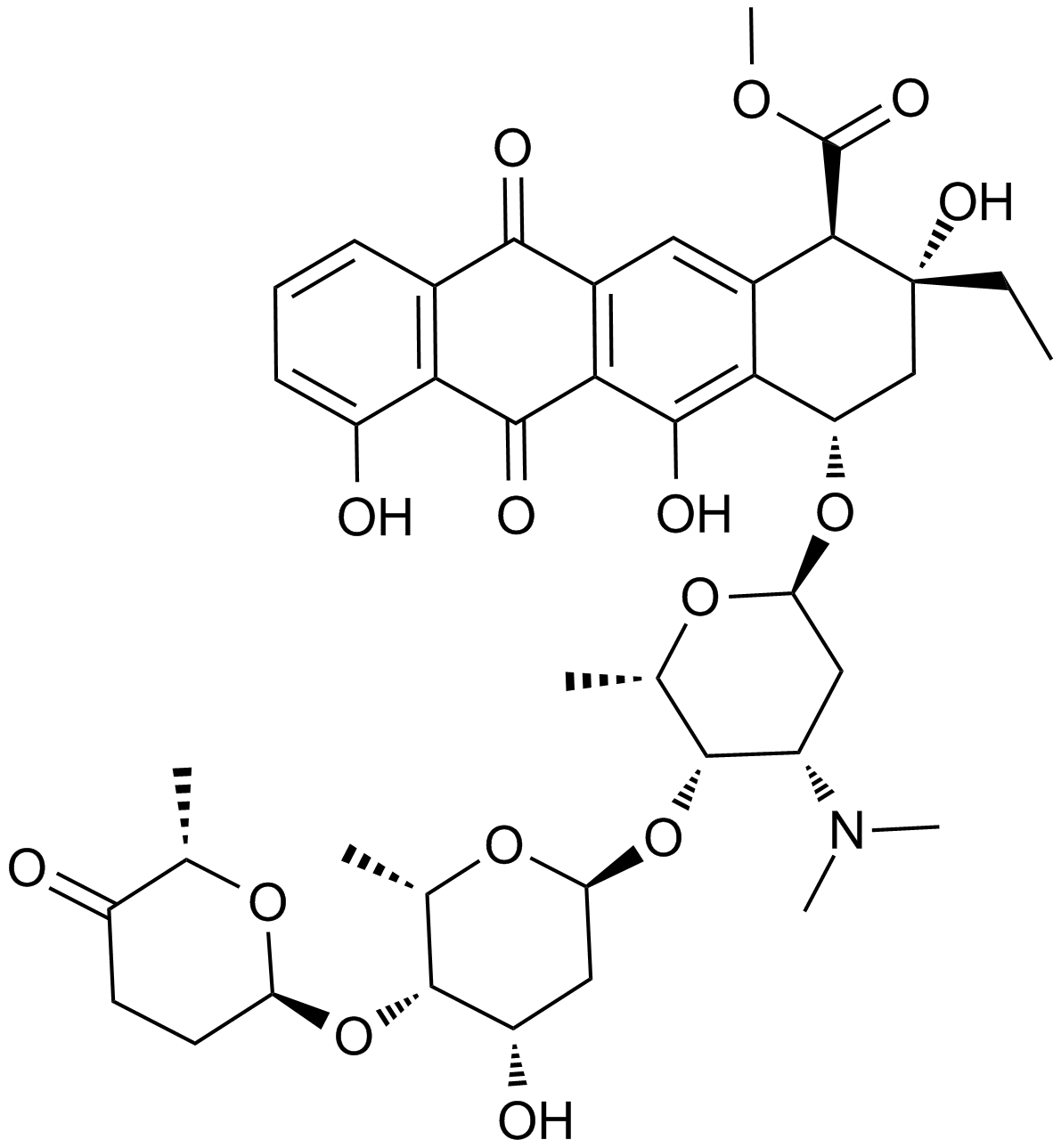 A2601 Aclacinomycin ATarget: TopoisomerasesSummary: Topoisomerase I and II inhibitor
A2601 Aclacinomycin ATarget: TopoisomerasesSummary: Topoisomerase I and II inhibitor -
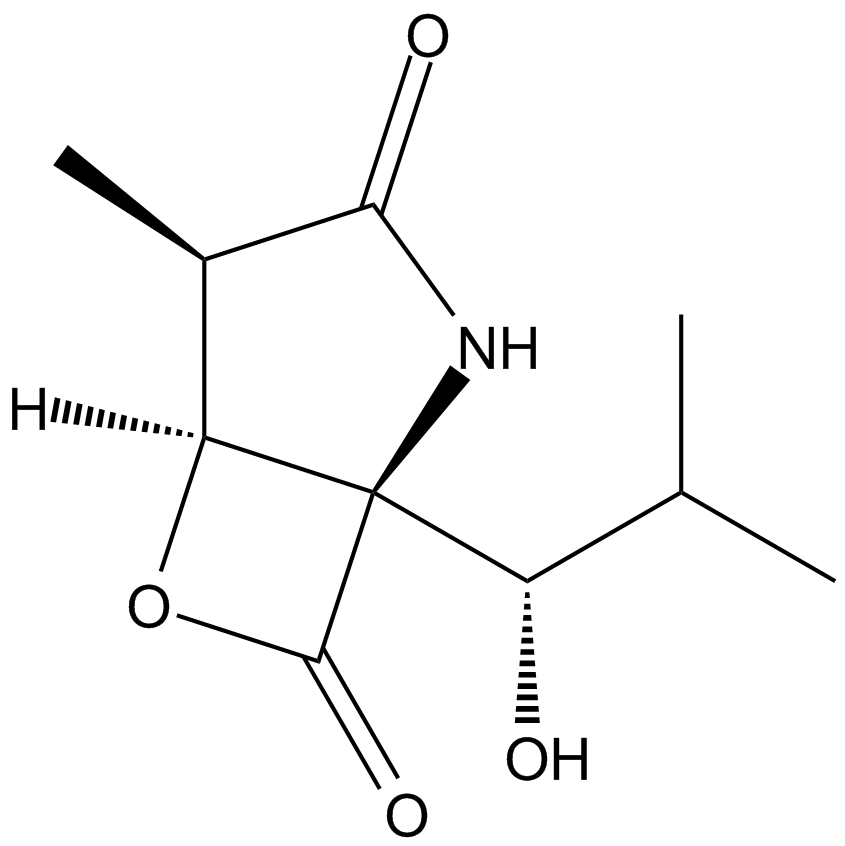 A2578 Clasto-Lactacystin β-lactoneTarget: ProteasomeSummary: Proteasome inhibitor
A2578 Clasto-Lactacystin β-lactoneTarget: ProteasomeSummary: Proteasome inhibitor -
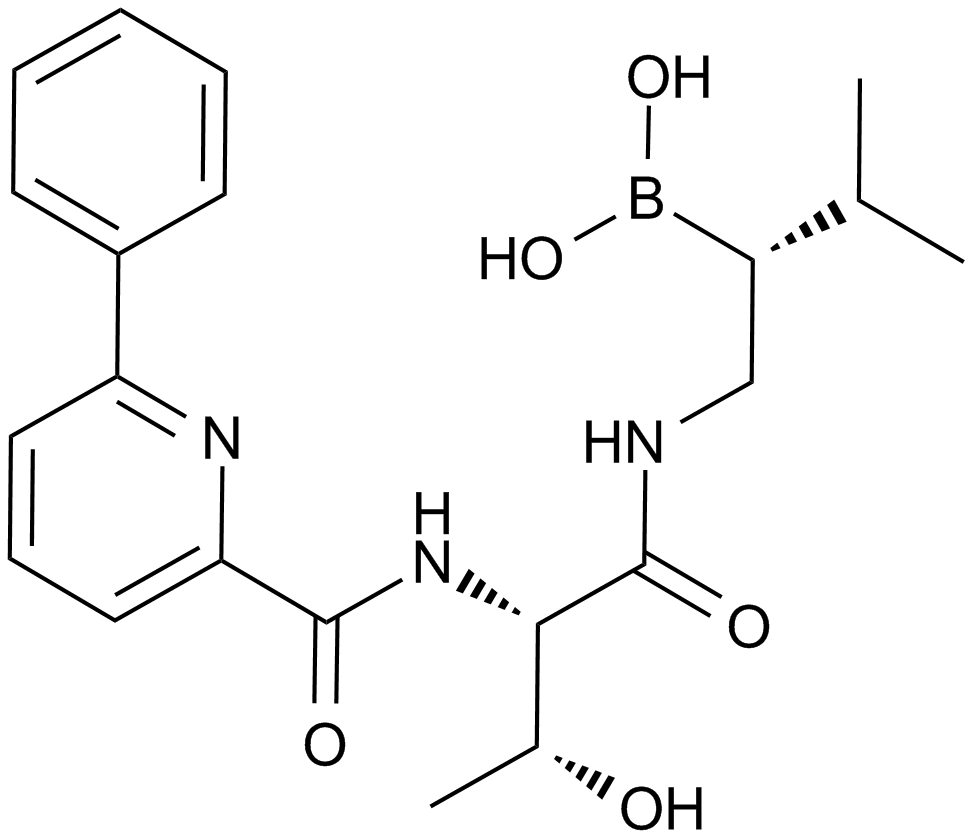 A4009 CEP-187701 CitationSummary: Proteasome inhibitor
A4009 CEP-187701 CitationSummary: Proteasome inhibitor -
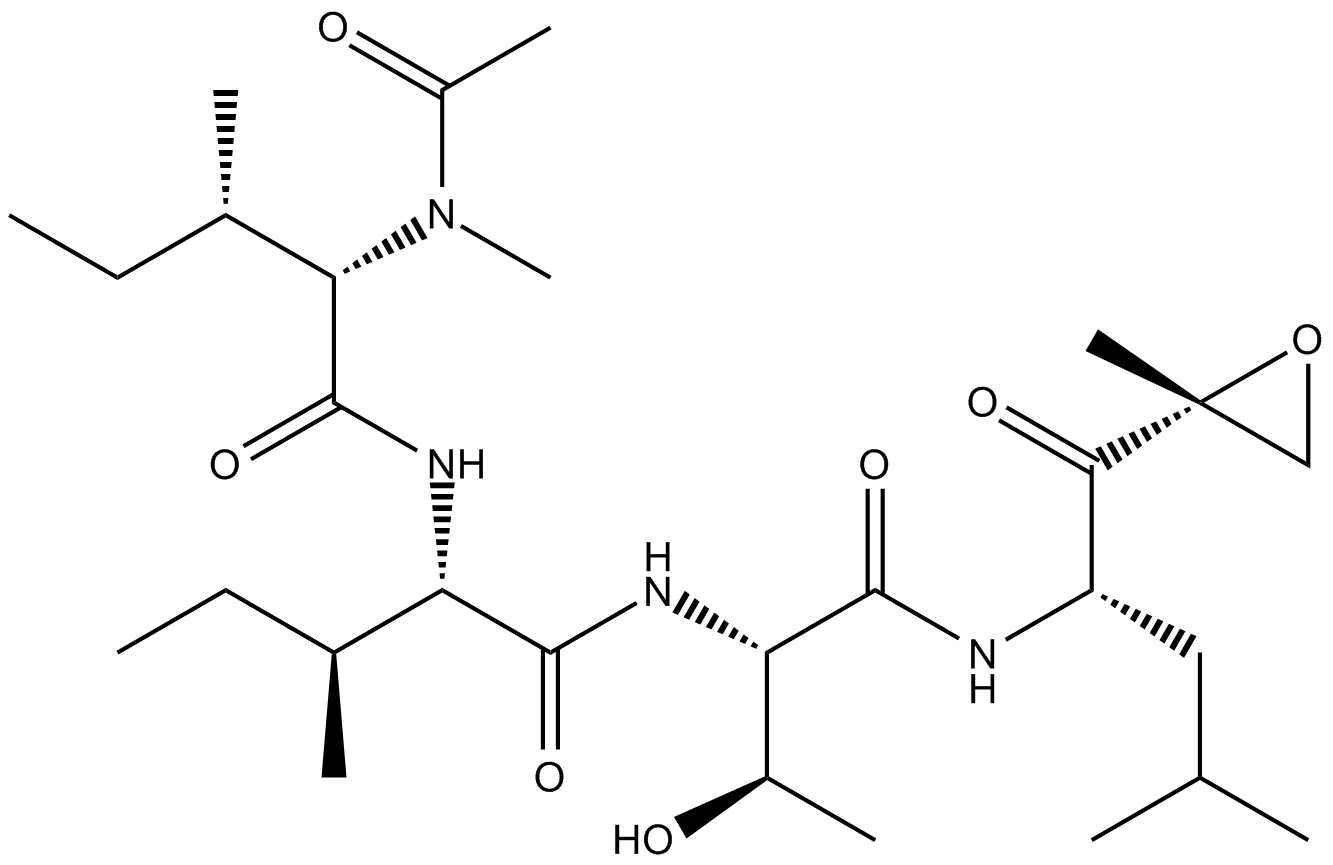 A2606 Epoxomicin25 CitationSummary: Proteasome inhibitor
A2606 Epoxomicin25 CitationSummary: Proteasome inhibitor -
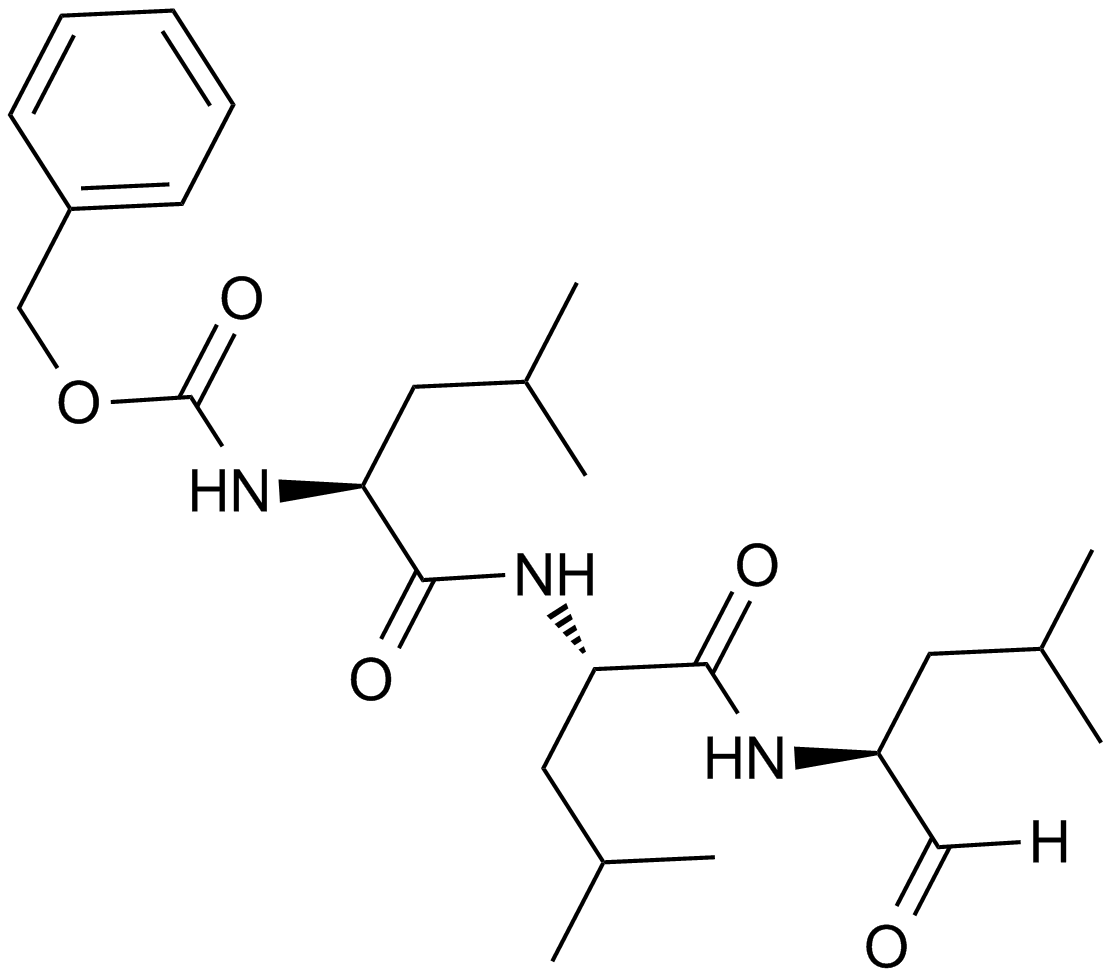 A2585 MG-13226 CitationTarget: ProteasomeSummary: Proteasome inhibitor, Cell permeable, reversible
A2585 MG-13226 CitationTarget: ProteasomeSummary: Proteasome inhibitor, Cell permeable, reversible -
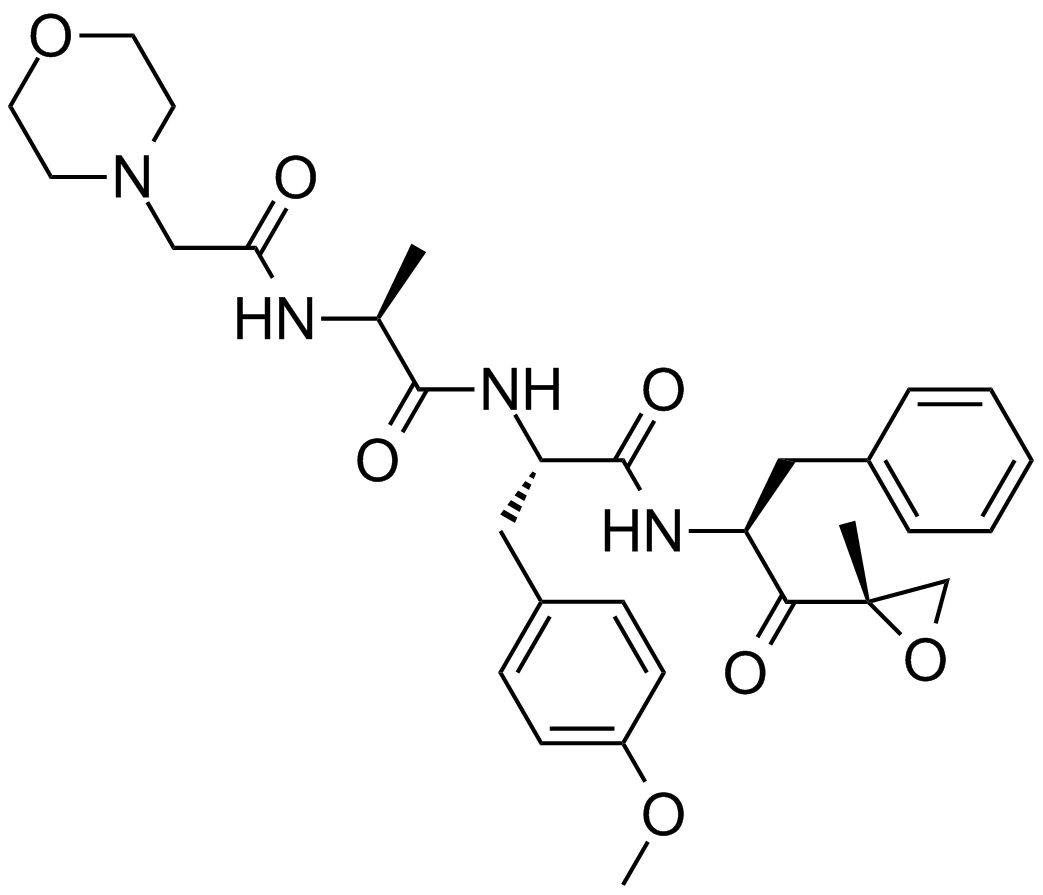 A4011 ONX-0914 (PR-957)6 CitationTarget: ProteasomeSummary: Immunoproteasome inhibitor,potent and selective
A4011 ONX-0914 (PR-957)6 CitationTarget: ProteasomeSummary: Immunoproteasome inhibitor,potent and selective -
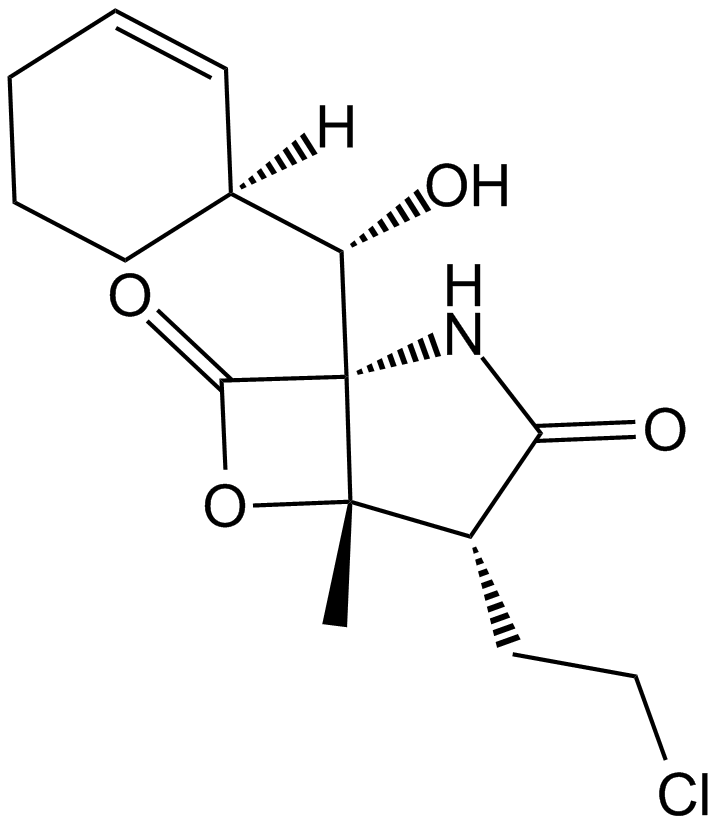 A4010 Salinosporamide A (NPI-0052, Marizomib)Target: ProteasomeSummary: 20S proteasome inhibitor
A4010 Salinosporamide A (NPI-0052, Marizomib)Target: ProteasomeSummary: 20S proteasome inhibitor

