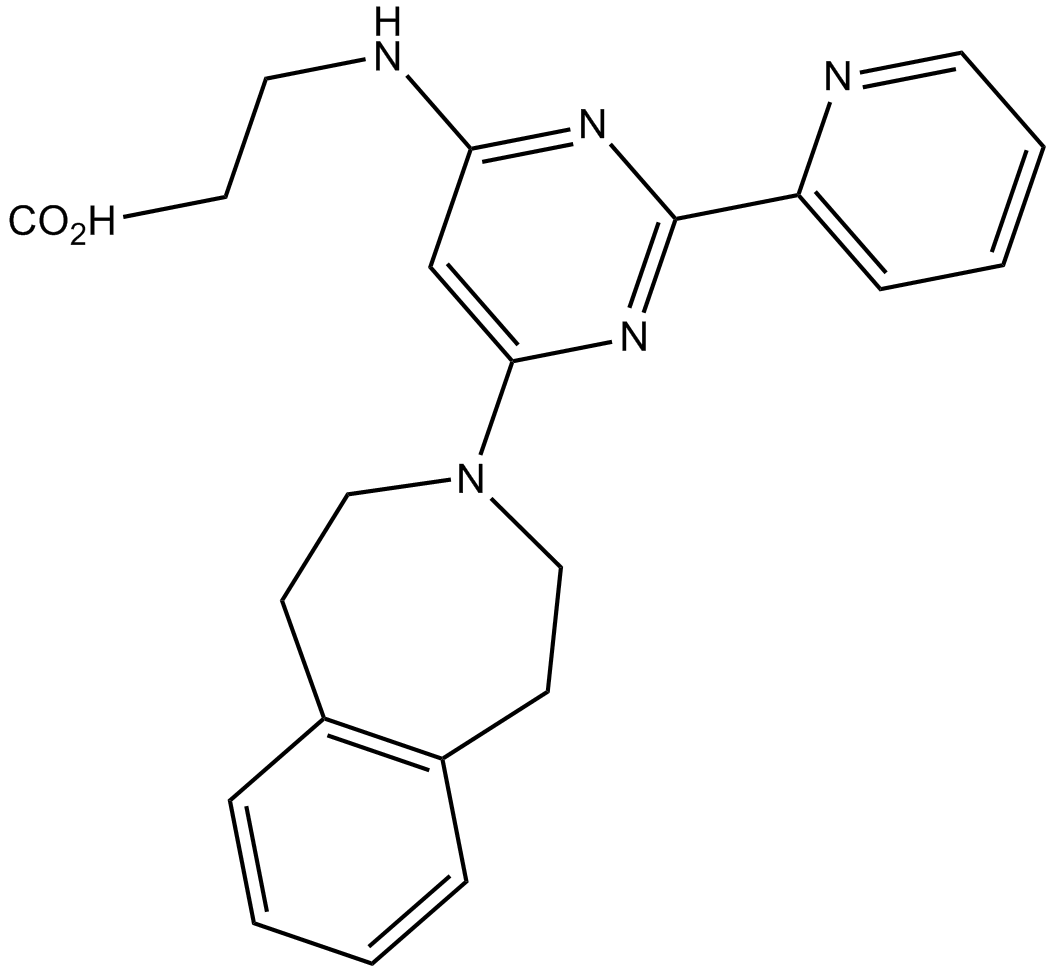
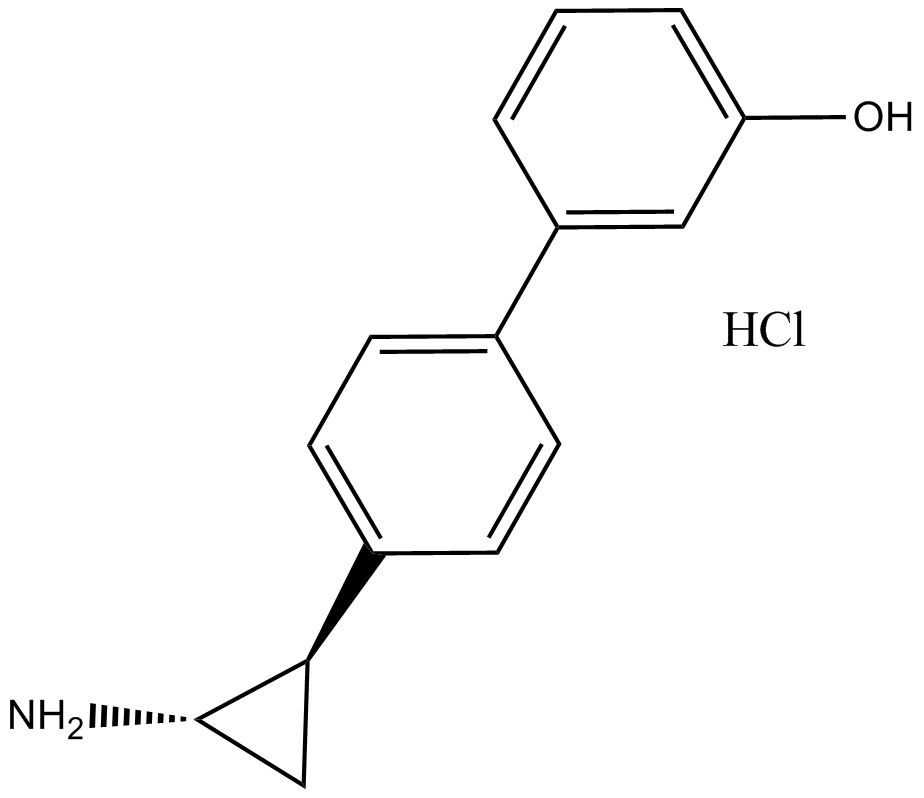
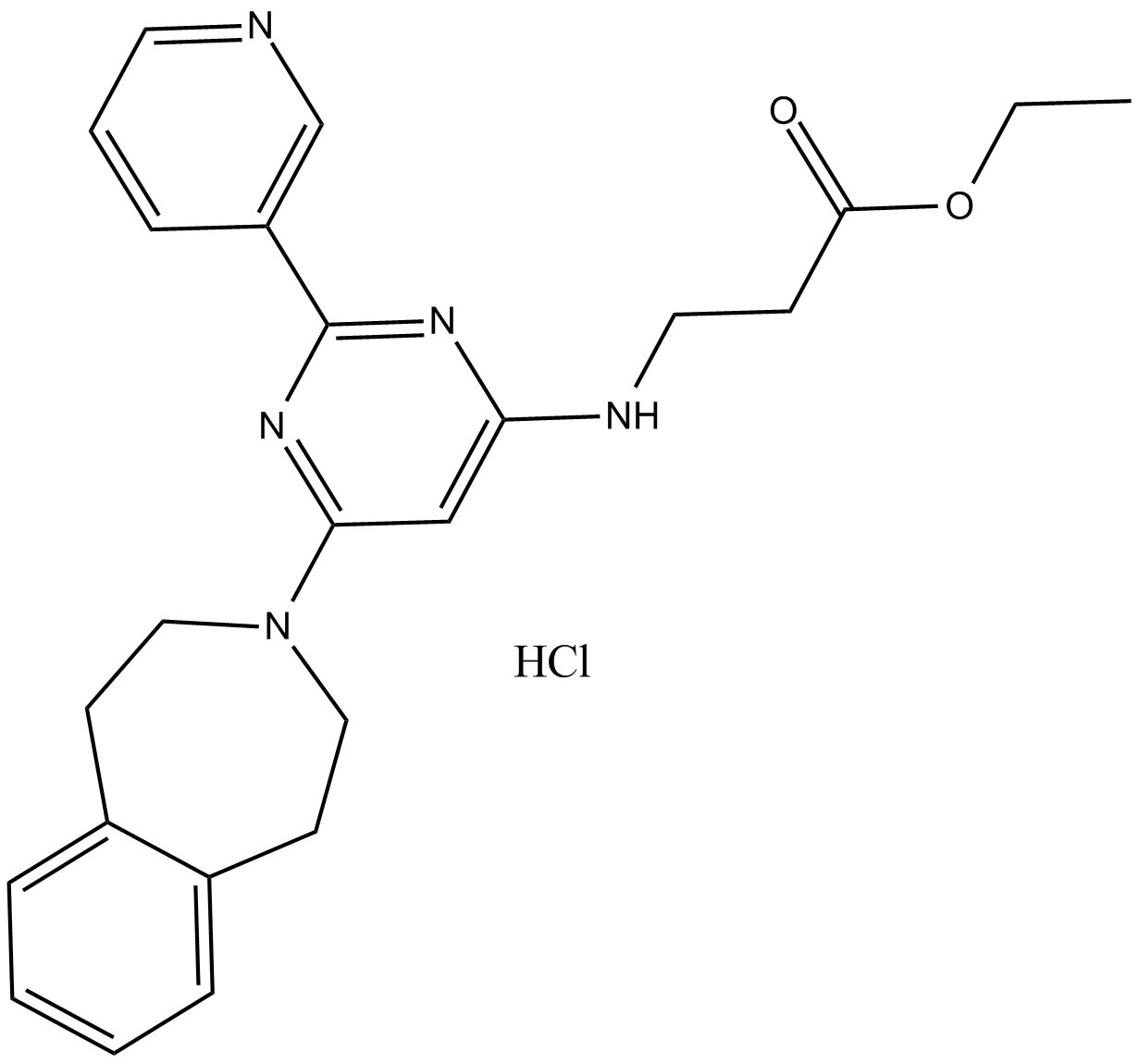
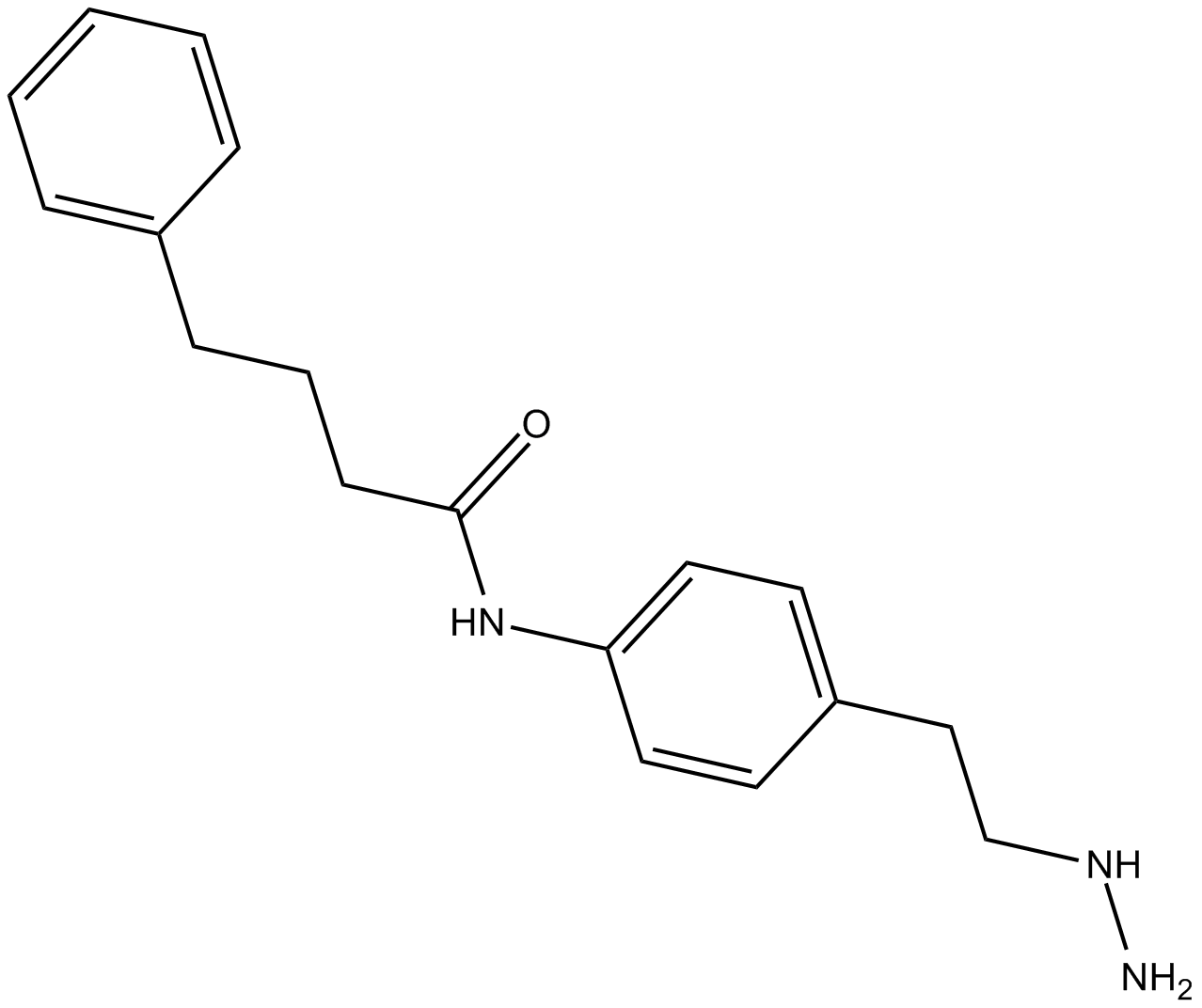
Histone Demethylases
Histone demethylases are a diverse group of enzymes catalyzing a process to reverse histone methylation, in which methyl (CH3-) groups are removed from instead of being transferred to histone. So far, two evolutionarily conserved histone demethylase families have been identified, including LSD demethylases and JMJC demethylases. LSD1 and LSD2 are two well-established members of LSD demethylase family, which catalyze demethylation through a flavin adenine dinucleotide (FAD)-dependent amine oxidation reaction. LSD1 has been found to demethylate H3K4mel, H3K4me2, H3K9me1, H3K9me2 and a few non-histone targets; while LSD2 has been found to demethylate only H3K4me1 and H3K4me2. Members of JMJC demethylase family is characterized by containing the catalytic JMJC domain, which demethylate substrates, including H3K4, H3K9, H3K27, H3K36 and H4K20, through a Fe(II)- and α-ketogutarate-dependent dioxygenase reaction.