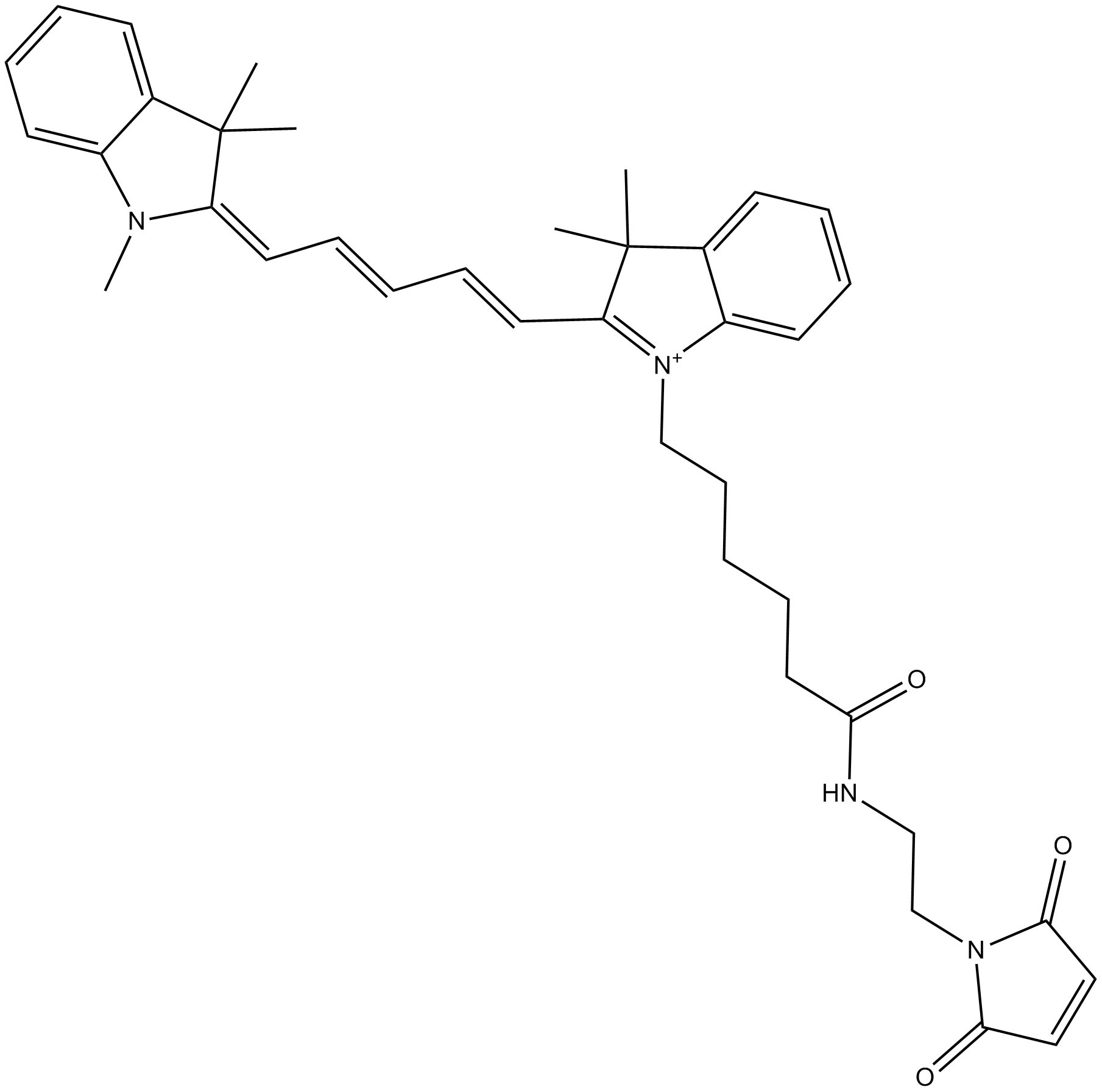
Cyanine dyes
Cyanine dyes are molecules containing polymethine bridges between two nitrogen atoms with a delocalized
Non-sulfonated cyanines
Available non-sulfonated dyes include Cy3, Cy3.5, Cy5, Cy5.5, Cy7, and Cy7.5. Cy stands for 'cyanine', and the first digit identifies the number of carbon atoms between the indolenine groups. The suffix .5 is added for benzo-fused cyanines. Most derivatives of non-sulfonated cyanines have low aqueous solubility except for hydrochlorides of hydrazides and amines. They are organic co-solvent soluble (5-20% of DMF or DMSO). When these molecules are used for biomolecule labeling, they should be dissolved in organic solvent first, and added to a solution of biomolecule (protein, peptide, amino-labeled DNA) in appropriate aqueous buffer. Fluorescent properties of non-sulfonated cyanines have little dependence on solvent and surrounding.
Sulfonated cyanines
Available sulfonated cyanines include sulfo-Cy3, sulfo-Cy5, and sulfo-Cy7, which have additional sulfo-groups that facilitate dissolution of dye molecules in aqueous phase. Charged sulfonate groups decrease aggregation of dye molecules and heavily labeled conjugates. Sulfonated cyanines are highly water soluble, and they do not use organic co-solvent for the labeling in aqueous environment.