EZ Cap™ Firefly Luciferase mRNA (5-moUTP)
EZ Cap™ Firefly Luciferase mRNA (5-moUTP) will express luciferase protein once entering cells, which is initially extracted from firefly Photinus pyralis. This enzyme catalyzes ATP-dependent D-luciferin oxidation and lead to yield chemiluminescence at about 560 nm wavelength. Firefly Luciferase is a frequently used bioluminescent reporter for gene regulation and function study. It is applicable in assays for mRNA delivery, translation efficiency, cell viability and in vivo imaging etc.
Key features:
Cap1 Structure: The mRNA incorporates a Cap1 analog at the 5' end, ensuring high translation initiation efficiency, improved stability, and reduced innate immune recognition, leading to stronger and more sustained luciferase expression.
Modified Nucleotides: It contains 5-methoxyuridine (5-moU) modified nucleotides, which decreases immunogenicity, enhances mRNA stability, and increases translational efficiency, resulting in more reliable and reproducible protein yield.
poly(A) tail: It contains an optimized poly(A) tail of approximately 100 nucleotides. This specific length is engineered to maximize transcript stability by resisting degradation and to synergize with the 5' cap for superior, sustained translation efficiency, ensuring robust protein yield in your applications.
| mRNA Length | 1921 nucleotides | ||
| Concentration | 1 mg/mL | ||
| Buffer | 1 mM Sodium Citrate, pH 6.4 | Storage | -40°C or below |
| General tips | Please dissolve it on ice and protect from RNase carefully. Avoid repeated freeze/thaw cycles as possible. Don’t vortex. Upon first use, centrifuge the tube softly and aliquot it into several single use portions. Use RNase-free reagents and materials with appropriate RNase-free technique. Don’t add to the media with serum unless mixing with a transfection reagent. | ||
| Shipping Condition | ship with dry ice. | ||
- 1. Blerina Shkodra, Ashish Muglikar, et al. "Boosting LNP Performance: Higher Concentrations of Lipid Mixtures Improve In Vivo Gene Expression and Storage Stability." Pharmaceutics 2026, 18(1), 50
- 2. Weixiang Gao, Kang An, et al. "Resolving the mRNA Encapsulation-Release Trade-off via Compensatory Forces in Engineered Ionizable Lipids." Adv Mater. 2025 Nov 16:e12235. PMID: 41243603
- 3. Ruiteng Song, Yongqi Lin, et al. "Ocular delivery of lipid nanoparticles-formulated mRNA encoding lanosterol synthase ameliorates cataract in rats." Nat Commun. 2025 Sep 26;16(1):8522. PMID: 41006301
- 4. Zhenghua Li, Jiacai Wu, et al. "mRNA-LNP hydrogels promote skeletal muscle regeneration in situ." Journal of Controlled Release Available online 23 September 2025, 114258. PMID: 40997951
- 5. Desheng Cao, Junliang Zhu, et al. "Dynamically covalent lipid nanoparticles mediate CRISPR-Cas9 genome editing against choroidal neovascularization in mice." Sci Adv. 2025 Jul 11;11(28):eadj0006. PMID: 40644543
- 6. Burcu Binici, Zahra Rattray, et al. "A comparative study of cationic lipid-enriched LNPs for mRNA vaccine delivery." Int J Pharm. 2025 Sep 15:682:125941. PMID: 40623607
- 7. Kai V. Slaughter, Daniela Isaacs-Bernal, et al. "RNA lipid nanoparticles stabilized during nebulization through excipient selection." Nanoscale Adv. 2025 Jun 3;7(14):4480-4489. PMID: 40519236
- 8. Jade Forrester, Callum G. Davidson, et al. "Low-Cost Microfluidic Mixers: Are They up to the Task?." Pharmaceutics. 2025 Apr 25;17(5):566. PMID: 40430858
- 9. Ankita Borah, Valeria Giacobbo, et al. "From in vitro to in Vivo: The Dominant role of PEG - Lipids in LNP performance." Eur J Pharm Biopharm. 2025 Jul:212:114726. PMID: 40286880
- 10. Jin-Yue Zeng, Shonya Lingesh, et al. "Cholesterol‐Derived Mannosylated Polypeptide‐Formed Lipid Nanoparticles for Efficient in Vivo mRNA Delivery." Small Methods. 2025 Jun;9(6):e2401712. PMID: 40256901
- 11. Zejuan Liu, Chen Chen, et al. "Legumain In Situ Engineering Promotes Efferocytosis of CAR Macrophage to Treat Cardiac Fibrosis." Adv Mater. 2025 Apr 14:e2417831. PMID: 40223483
- 12. Heyun Wang, Hugo Pestre, et al. "Facile lipid nanoparticle size engineering approach via controllable fusion induced by depletion forces." J Colloid Interface Sci. 2025 Aug:691:137334. PMID: 40147373
- 13. Chuanmei Tang, Yexi Zhang, et al. "Modular Design of Lipopeptide‐Based Organ‐Specific Targeting (POST) Lipid Nanoparticles for Highly Efficient RNA Delivery." Adv Mater.2025 Mar;37(11):e2415643. PMID: 39924757
- 14. Menghua Gao, Jiafeng Zhong, et al. "Deciphering the Role of PEGylation on the Lipid Nanoparticle-Mediated mRNA Delivery to the Liver." ACS Nano. 2025 Feb18;19(6):5966-5978. PMID: 39899798
- 15. Jian Hang Lam, Gaurav Sinsinbar, et al. "Development of Thermostable and Immunogenic Block Copolymer Nanoparticles (BNPs) for mRNA Delivery." Biomacromolecules. 2025 Apr 14;26(4):2444-2457
- 16. Songbo Zhao, Rongkun Li, et al. "Targeting ECM-producing cells with CAR-T therapy alleviates fibrosis in chronic kidney disease." Cell Stem Cell. 2025 Aug 18:S1934-5909(25)00271-1
- 17. Kai V. Slaughter, Eric N. Donders, et al. "Ionizable Drugs Enable Intracellular Delivery of Co-Formulated siRNA." Adv Mater. 2024 Oct;36(41):e2403701. PMID: 39148215
- 18. Burcu Binici, Zahra Rattray, et al. "The Role of Biological Sex in Pre-Clinical (Mouse) mRNA Vaccine Studies." Vaccines (Basel). 2024 Mar 7;12(3):282. PMID: 38543916
- 19. Shiguo Zhu, et al. "Euphohelioscopin A enhances NK cell antitumor immunity through GSDME-triggered pyroptosis." J Leukoc Biol. 2024 Mar 8:qiae055. PMID: 38456763
- 20. Zhicheng Le, Jiang Qian, et al. "A versatile gemini amphiphile-based platform with STING-activating properties for efficient gene delivery into dendritic cells." Chemical Engineering Journal Volume 497, 1 October 2024, 154513
- 21. Zepeng He, Zhicheng Le, et al. "A Multidimensional Approach to Modulating Ionizable Lipids for High-Performing and Organ-Selective mRNA Delivery." Angew Chem Int Ed Engl. 2023 Oct 23;62(43):e202310401. PMID: 37661193
- 22. Min Tang, Ayane Sagawa, et al. "Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method." Pharmaceutics. 2023 Apr 4;15(4):1141. PMID: 37111627
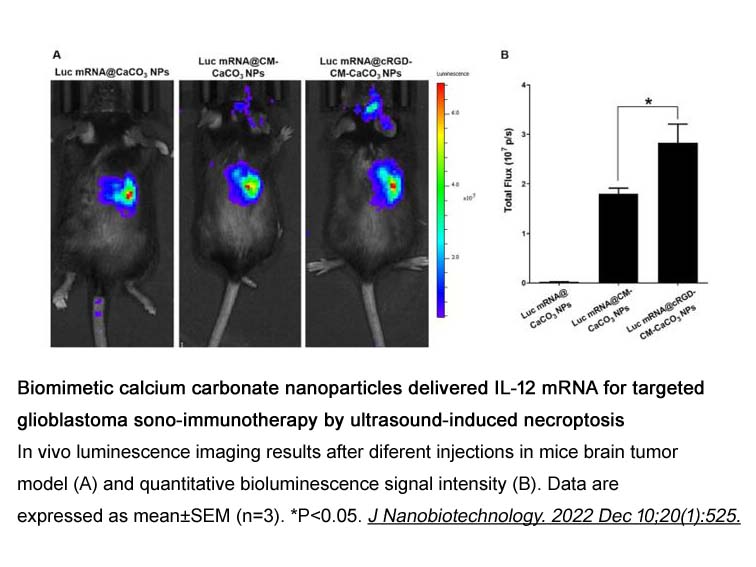
- 23. Pengxuan Zhao, Yu Tian, et al. "Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis." J Nanobiotechnology. 2022 Dec 10;20(1):525. PMID: 36496387
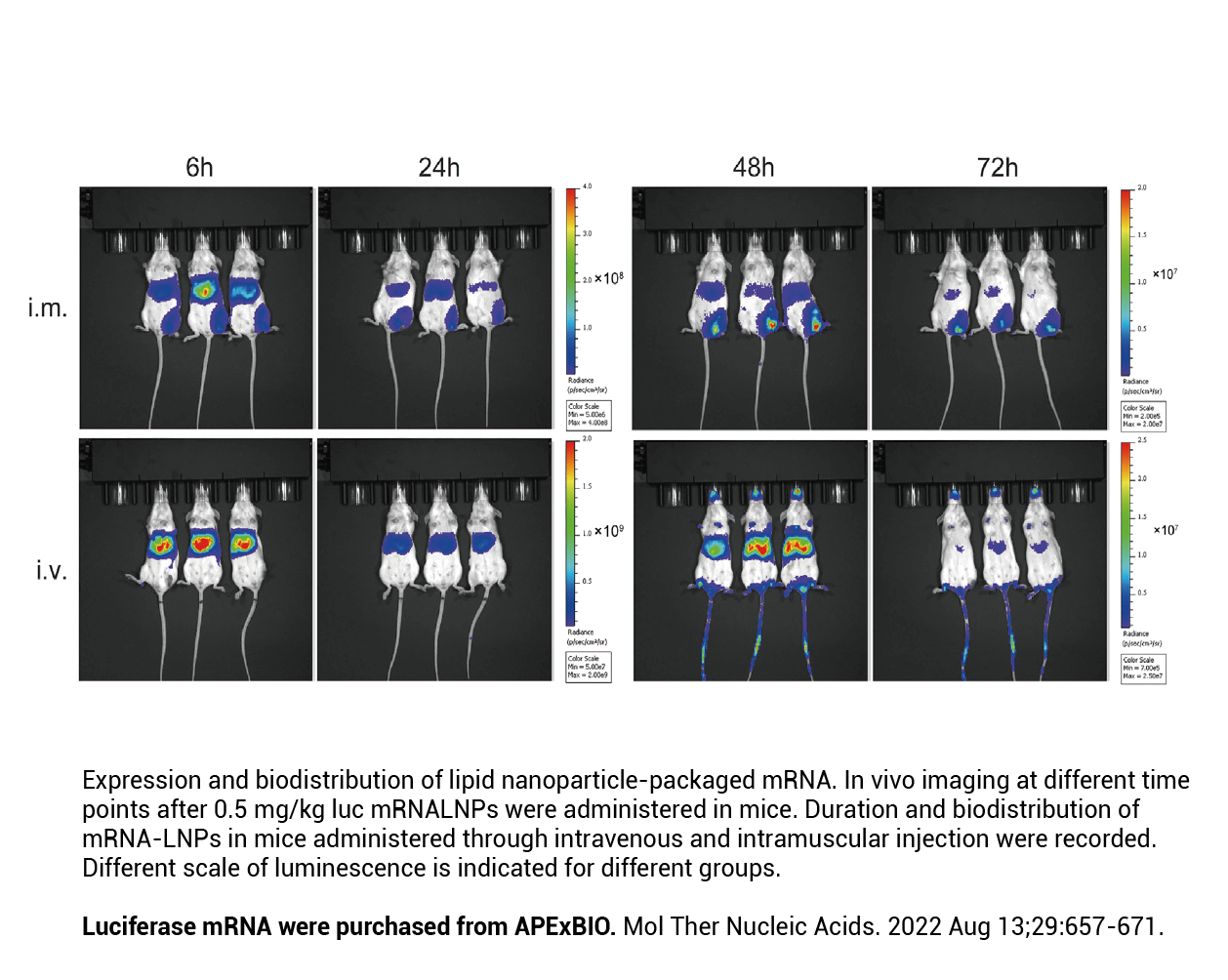
- 24. Yu Zhang, Xiaodong Xi, et al. "Chemically modified in-vitro-transcribed mRNA encoding thrombopoietin stimulates thrombopoiesis in mice." Mol Ther Nucleic Acids. 2022 Aug 13;29:657-671. PMID: 36090760
Quality Control & Datasheet
- View current batch:
Related Biological Data

Related Biological Data
Related Biological Data
It can be based on your experimental goals:
- For Tracking Transfection and Translation Efficiency: APExBIO Reporter Gene mRNAs (e.g. EGFP, Firefly Luciferase mRNA) are commonly used to track transfection efficiency and protein expression duration; evaluate gene expression and cell viability; study mRNA localization and bio-distribution via in vivo imaging; optimize transfection conditions and validate LNP delivery system.
- For Gene Editing, Functional Studies and Gene Therapy Research: APExBIO offers various functional protein mRNAs, involving tumor suppressors (e.g. p53, PTEN), cytokines (e.g. IL-12, IL-10), gene-editing tools (e.g. spCas9, Cre Recombinase), gene replacement protein (e.g. EPO), and antigens (e.g. OVA, SARS-CoV-2 Spike).
- For Sustained Protein Expression: APExBIO Self-amplifying RNA (saRNA) and Circular RNA (circRNA) are recommended for applications requiring prolonged protein expression. saRNA enables lasting and strong protein expression at lower doses, while circRNA has enhanced structural stability and extended expression duration.
- Advanced Capping Technology: Utilizes Cap 1 structure (EZ Cap™ Cap) to achieve enhanced translation efficiency and minimizing activation of the host innate immune response. The capping efficiency can reach 90–99%.
- Diverse Modification Options: Provides a range of modified nucleotides, such as m1Ψ, 5-moUTP and Cy5-UTP, which reduce immunogenicity, improve mRNA stability, and maximize protein expression levels.
- Stringent Quality Control: Each batch undergoes rigorous quality assessment including capping efficiency, purity, integrity, and sterility to ensure batch-to-batch consistency.