EZ Cap™ Cy5 Firefly Luciferase mRNA (5-moUTP)
EZ Cap™ Cy5 Firefly Luciferase mRNA (5-moUTP) is a high-performance, dual-reporting mRNA product designed for advanced gene expression and intracellular tracking studies. It combines robust protein expression with sensitive fluorescence detection in a single construct. Firefly Luciferase catalyzes ATP-dependent D-luciferin oxidation and lead to yield chemiluminescence at about 560 nm wavelength. Cy5 (Cyanine-5) is a fluorescent compound with an excitation peak at 646 nm and an emission peak at 662 nm. This ready-to-use mRNA is ideal for real-time tracking of mRNA delivery, optimizing transfection protocols, and dual-modality studies combining bioluminescence imaging with fluorescence-based localization. It serves as a valuable tool in mRNA vaccine development, gene therapy research, and intracellular trafficking assays.
Key features:
Fluorescent Labeling: The mRNA is covalently conjugated with Cy5 dye, enabling direct visualization of mRNA delivery, cellular uptake, and intracellular trafficking using fluorescence microscopy or flow cytometry, without the need for secondary detection steps.
Cap1 Structure: The mRNA incorporates a Cap1 analog at the 5' end, ensuring high translation initiation efficiency, improved stability, and reduced innate immune recognition, leading to stronger and more sustained luciferase expression.
Modified Nucleotides: It contains 5-methoxyuridine (5-moU) modified nucleotides, which decreases immunogenicity, enhances mRNA stability, and increases translational efficiency, resulting in more reliable and reproducible protein yield.
| mRNA Length | 1921 nucleotides |
| Concentration | 1 mg/mL |
| Buffer | 1 mM Sodium Citrate, pH 6.4 |
| Storage | -40°C or below |
| General tips | Dissolve it on ice and take care to prevent RNase contamination degradation. Avoid repeated freezing and thawing as much as possible. Do not vortex. For the first time, it is gently centrifuged and divided into several parts for stand-alone use. Use RNase-free reagents and consumables, using appropriate RNase-free technology. It can not be added to the serum-containing medium until it is mixed with the transfection reagent. |
| Shipping Condition | Ship with dry ice. |
- 1. Shuling Ren, Xinyu Lin, et al. "Redox-Responsive Peptide Coacervates for Enhanced mRNA Delivery and Intracellular Release." ACS Nano. 2025 Dec 26. PMID: 41451635
- 2. Paul Folda, Eric Weidinger, et al. "PEGylation Enhances Colloidal Stability and Promotes Ligand-Mediated Targeting of LAF-Xenopeptide mRNA Complexes." Polymers (Basel). 2025 Nov 9;17(22):2979. PMID: 41304345
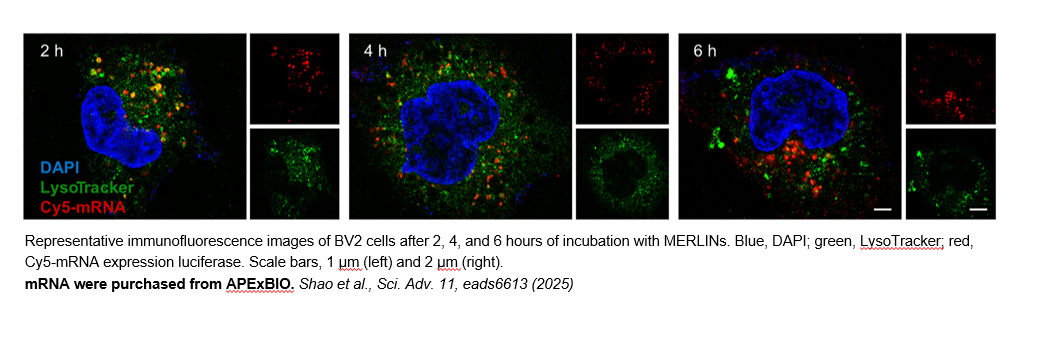
- 3. Lijuan Shao, Yi Zhang, et al. "Synthetic efferocytic receptor microglia enhances anti-inflammatory clearance of amyloid-β for AD treatment in mice." Sci Adv. 2025 Jul 11;11(28):eads6613. PMID: 40632863
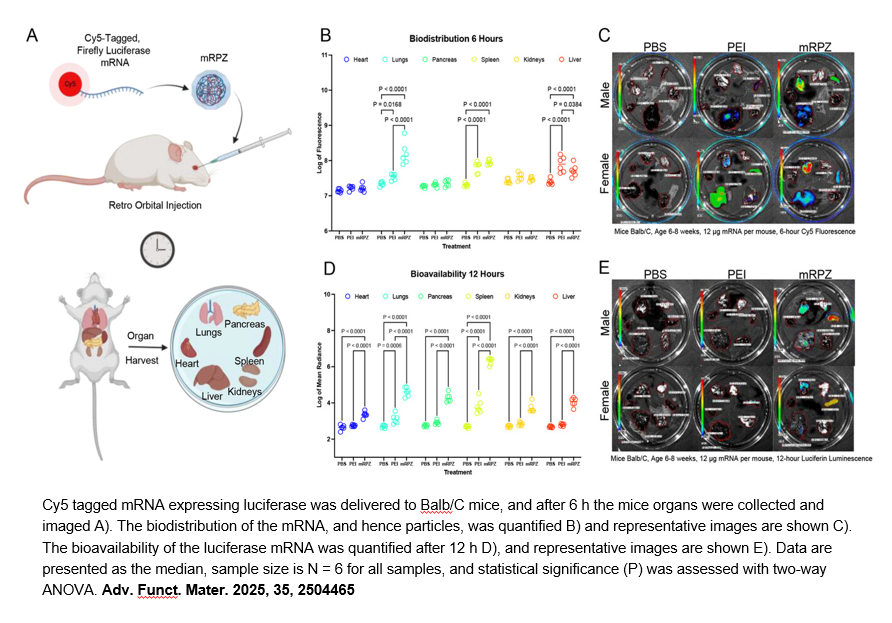
- 4. Harrison Douglas Lawson, Huy Hoang Nguyen, et al. "Synthetic Strategy for mRNA Encapsulation and Gene Delivery with Nanoscale Metal‐Organic Frameworks." ADVANCED FUNCTIONAL MATERIALS 19 May 2025
- 5. Jade Forrester, Callum G. Davidson, et al. "Low-Cost Microfluidic Mixers: Are They up to the Task?." Pharmaceutics. 2025 Apr 25;17(5):566. PMID: 40430858
- 6. Dao Thi Hong Le, Chuan Yang, et al. "Replacing PEG-Lipid with Amphiphilic Polycarbonates in mRNA-Loaded Lipid Nanoparticles: Impact of Polycarbonate Structure on Physicochemical and Transfection Properties." Biomacromolecules. 2025 Jun 9;26(6):3563-3575. PMID: 40347133
- 7. Nipuni Maniyamgama, Ki Hyun Bae, et al. "Muco‐Penetrating Lipid Nanoparticles Having a Liquid Core for Enhanced Intranasal mRNA Delivery." Adv Sci (Weinh). 2025 Mar;12(11):e2407383. PMID: 39888252
- 8. Li Zhang, Brandon Yi Loong Seow, et al. "Role of PEGylated lipid in lipid nanoparticle formulation for in vitro and in vivo delivery of mRNA vaccines." J Control Release. 2025 Apr10:380:108-124. PMID: 39875076
- 9. Zixuan Zhen, Xinhao Lin, et al. "Impact of cell line and reporter gene selection on in-vitro transfection evaluation of mRNA lipid nanoparticles." AAPS Open 11, 20 (2025)06 October
- 10. Wenting Yang, Shangqian Li, Siqi Yu. "Combinatorial Discovery of RAFT Cationic Polymers for mRNA Delivery: Structure–Function Insights from High-Throughput Screening and Machine Learning." Biomacromolecules. 2025 Oct 6. PMID: 41053999
- 11. RYOHEI SHIMIZU, YOSHIYUKI HATTORI. "Effects of disaccharide and cationic lipid types on reverse transfection with lyophilized mRNA lipoplexes." EXPERIMENTAL AND THERAPEUTIC MEDICINE 30: 239, 2025
- 12. Yoshiyuki Hattori, Ryohei Shimizu et al. "Effective mRNA transfection of tumor cells using cationic triacyl lipid‑based mRNA lipoplexes." Biomed Rep. 2024 Dec 4;22(2):25. PMID: 39720303
- 13. Mohammed S Alqahtani, Rabbani Syed, et al. "Synthesis and bioactivity of a novel surfactin-based lipopeptide for mRNA delivery." Nanoscale Adv. 2024 Sep 4. PMID: 39247856
- 14. Runpu Ma, Yuting Li, et al. "The dynamic process of mRNA delivery by lipid nanoparticles in vivo." Nano Today Volume 57, August 2024, 102325
- 15. Min Tang, Yoshiyuki Hattori, et al. "Effect of vorinostat on protein expression in vitro and in vivo following mRNA lipoplex administration." Biomed Rep. 2024 May 27;21(1):105. PMID: 38868527
- 16. Franziska Haase, geb. Freitag aus Düsseldorf, et al. "A novel class of mRNA lipid nanoparticles (LNPs) optimized by the chemical evolution approach." 2024
- 17. Franziska Haase, Jana Pöhmerer, et al. "Lipoamino bundle LNPs for efficient mRNA transfection of dendritic cells and macrophages show high spleen selectivity." Eur J Pharm Biopharm. 2024 Jan:194:95-109. PMID: 38065313
- 18. Mingzhu Gao, Maoping Tang, et al. "Modulating Plaque Inflammation via Targeted mRNA Nanoparticles for the Treatment of Atherosclerosis." ACS Nano. 2023 Sep 26;17(18):17721-17739. PMID: 37669404
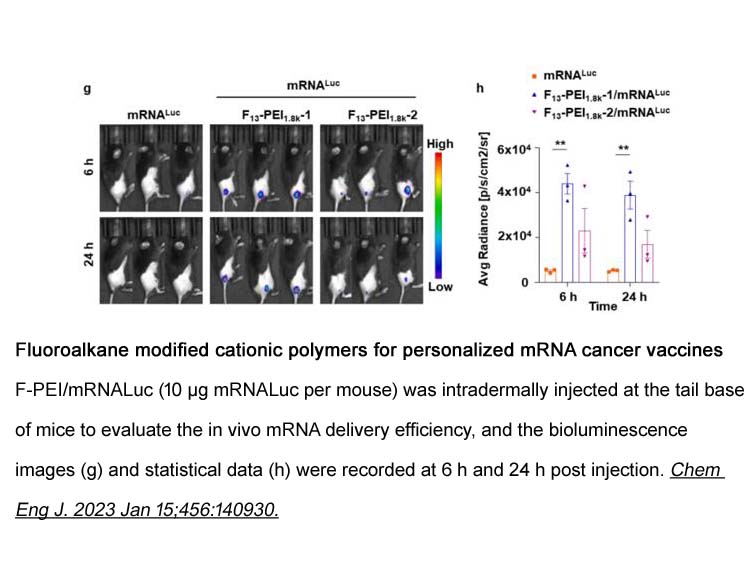
- 19. Junyan Li, Yuanyuan Wu, et al. "Fluoroalkane modified cationic polymers for personalized mRNA cancer vaccines." Chem Eng J. 2023 Jan 15:456:140930. PMID: 36531858
Quality Control & Datasheet
- View current batch:
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
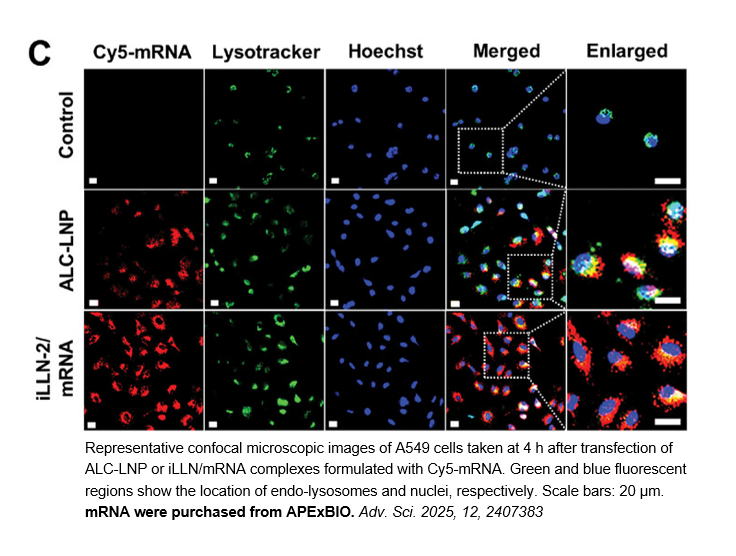
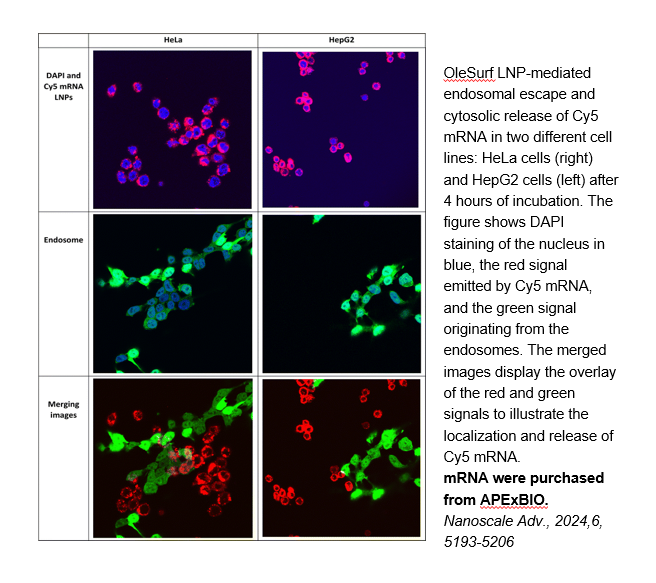
Related Biological Data
It can be based on your experimental goals:
- For Tracking Transfection and Translation Efficiency: APExBIO Reporter Gene mRNAs (e.g. EGFP, Firefly Luciferase mRNA) are commonly used to track transfection efficiency and protein expression duration; evaluate gene expression and cell viability; study mRNA localization and bio-distribution via in vivo imaging; optimize transfection conditions and validate LNP delivery system.
- For Gene Editing, Functional Studies and Gene Therapy Research: APExBIO offers various functional protein mRNAs, involving tumor suppressors (e.g. p53, PTEN), cytokines (e.g. IL-12, IL-10), gene-editing tools (e.g. spCas9, Cre Recombinase), gene replacement protein (e.g. EPO), and antigens (e.g. OVA, SARS-CoV-2 Spike).
- For Sustained Protein Expression: APExBIO Self-amplifying RNA (saRNA) and Circular RNA (circRNA) are recommended for applications requiring prolonged protein expression. saRNA enables lasting and strong protein expression at lower doses, while circRNA has enhanced structural stability and extended expression duration.
- Advanced Capping Technology: Utilizes Cap 1 structure (EZ Cap™ Cap) to achieve enhanced translation efficiency and minimizing activation of the host innate immune response. The capping efficiency can reach 90–99%.
- Diverse Modification Options: Provides a range of modified nucleotides, such as m1Ψ, 5-moUTP and Cy5-UTP, which reduce immunogenicity, improve mRNA stability, and maximize protein expression levels.
- Stringent Quality Control: Each batch undergoes rigorous quality assessment including capping efficiency, purity, integrity, and sterility to ensure batch-to-batch consistency.