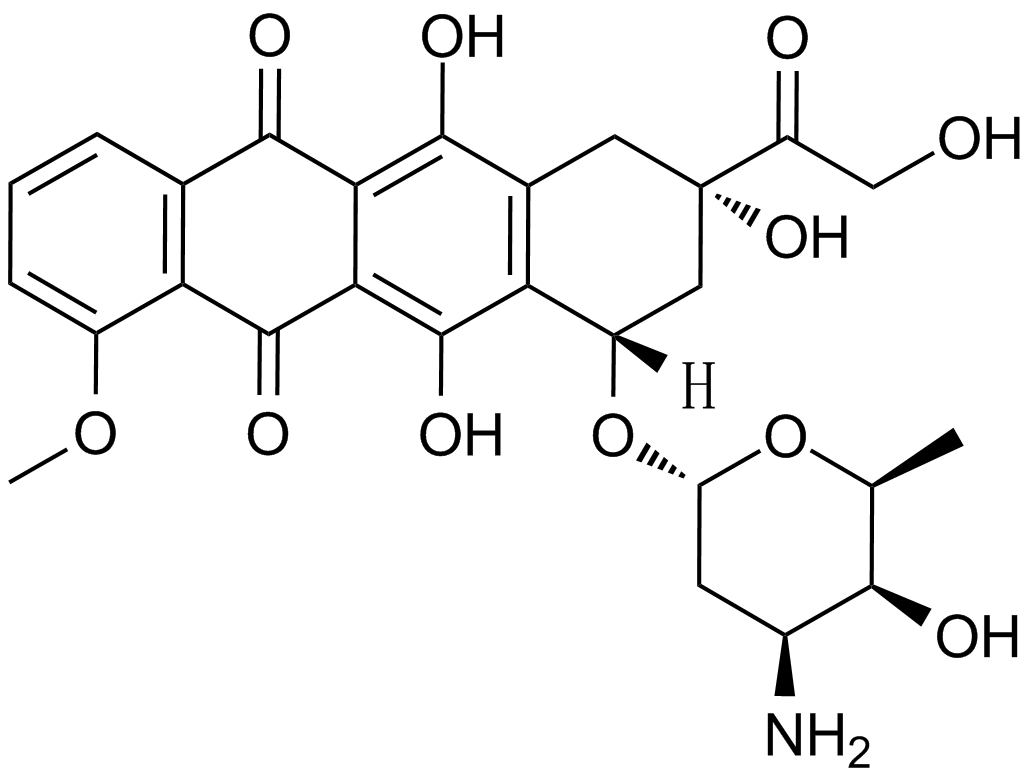
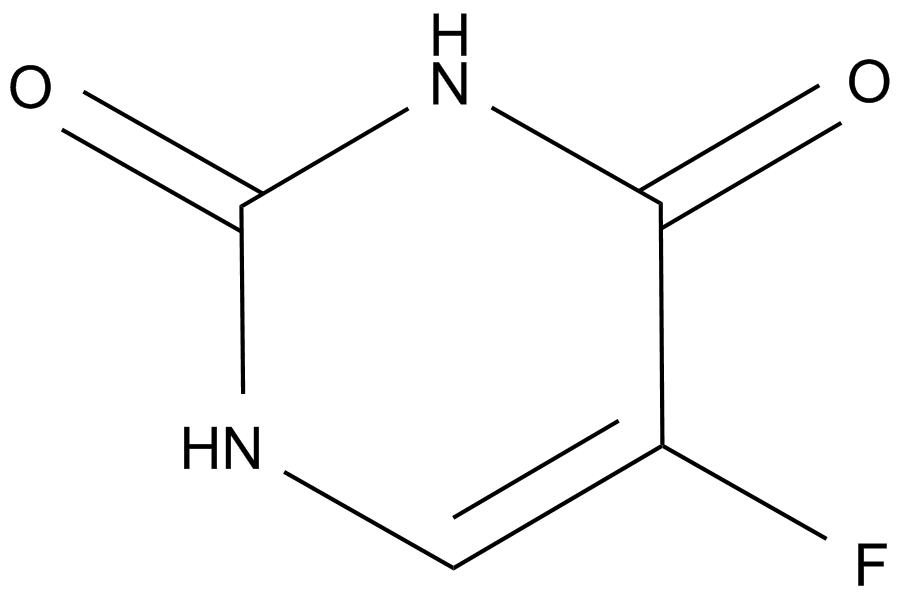
Fluorouracil (Adrucil)
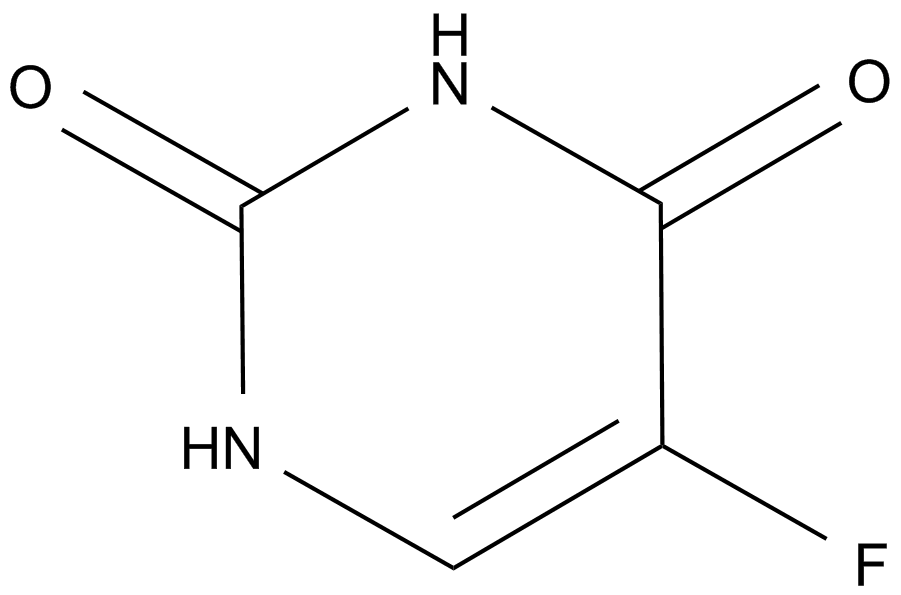
Fluorouracil (Adrucil), a heterocyclic aromatic organic compound, is a potent anticancer agent widely used for the treatment of solid tumors, including breast cancer, ovarian cancer, head and neck cancer, and colon cancer. As an analogue of uracil, fluorouracil has a fluorine atom replacing the hydrogen atom at the C-5 position. Due to its similar chemical structure to DNA and RNA, fluorouracil and metabolites exert strong anticancer activities through incorporation into DNA and RNA and inhibition of thymidylate synthase (TS). Fluorouracil is metabolized into fluorodeoxyuridine monophosphate (FdUMP), which inhibits TS by forming a stable complex with it and subsequently suppresses the production of deoxythymidine monophosphate (dTMP), an essential enzyme involved in DNA replication and repair, leading to cytotoxicity and cell death.
Reference
Ning Zhang, Ying Yin, Shen-Jie Xu and Wei-Shan Chen. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551-1569
Michael D. Wyatt and David M. Wilson III. Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci. 2009; 66(5): 788-799
- 1. Irit Shapira-Netanelov, Olga Furman, et al. "Patient-Derived Gastric Cancer Assembloid Model Integrating Matched Tumor Organoids and Stromal Cell Subpopulations." Cancers (Basel). 2025 Jul 9;17(14):2287 PMID: 40723172
- 2. Rongyao Liang, Pei Li, et al. "Parabacteroides distasonis-Derived Outer Membrane Vesicles Enhance Antitumor Immunity Against Colon Tumors by Modulating CXCL10 and CD8+ T Cells." Drug Des Devel Ther. 2024 May 28:18:1833-1853. PMID: 38828018
- 3. Libin Yan, Siyue Liu, et al. "Loss of SETD2‐mediated downregulation of intracellular and exosomal miRNA‐10b determines MAPK pathway activation and multidrug resistance in renal cancer." Mol Carcinog. 2023 Aug 17. PMID: 37589422
- 4. Zhengchun Wu, Ziru Liu, et al. "APEX1 predicts poor prognosis of gallbladder cancer and affects biological properties of CD133+ GBC-SD cells via upregulating Jagged1." J Cancer. 2023 May 15;14(8):1443-1457. PMID: 37283798
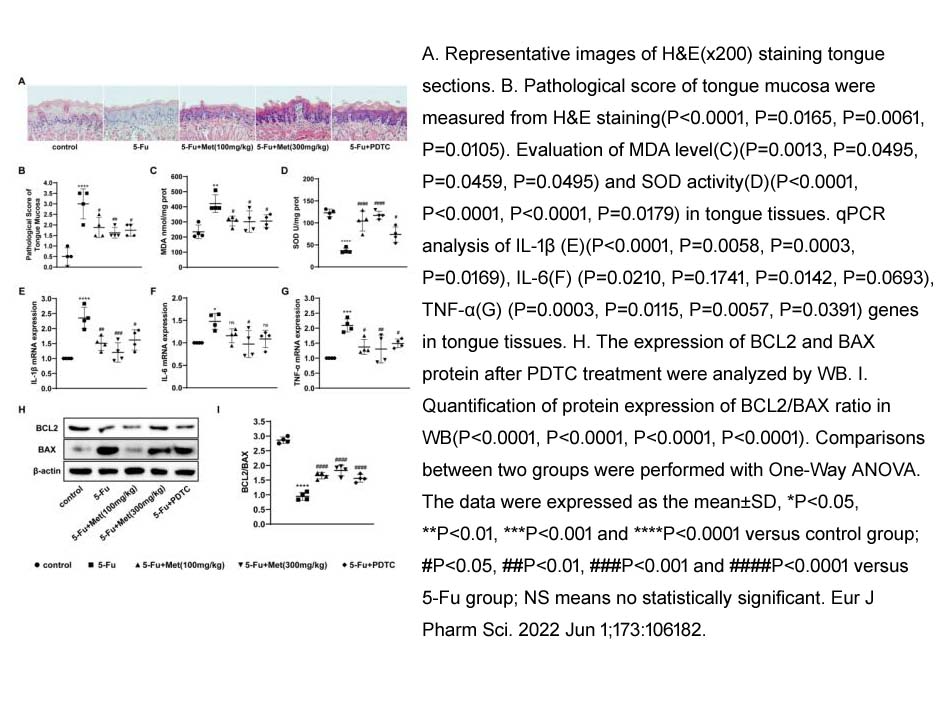
- 5. HangSun, YufengZhou, et al. "Metformin protects 5-Fu-induced chemotherapy oral mucositis by reducing endoplasmic reticulum stress in mice." Eur J Pharm Sci. 2022 Jun 1;173:106182. PMID: 35405270
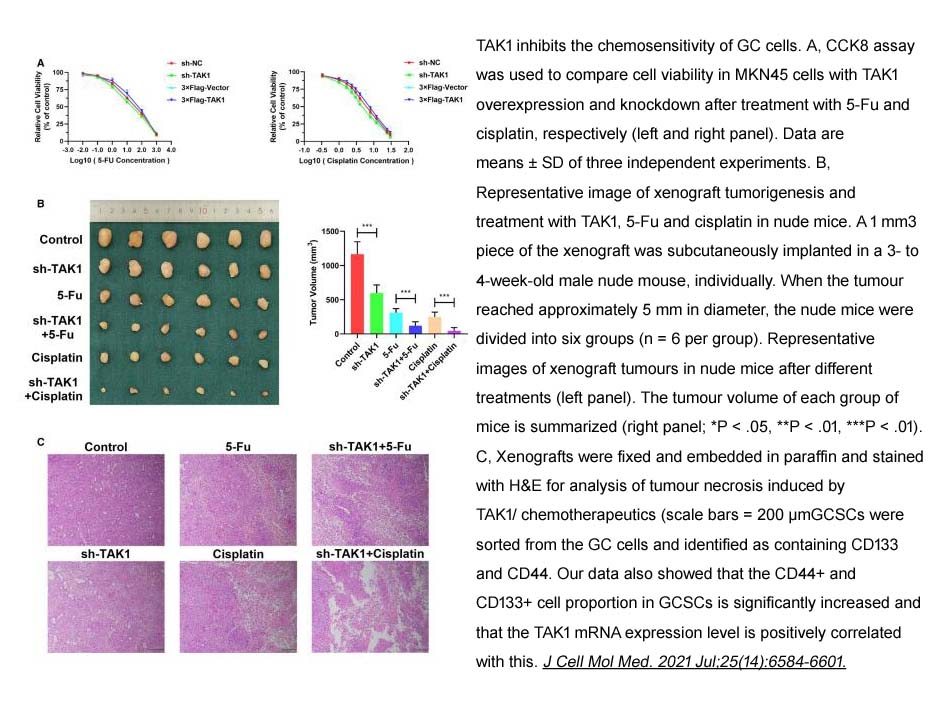
- 6. Gang Wang, Qikai Sun, et al. "The stabilization of yes-associated protein by TGFβ-activated kinase 1 regulates the self-renewal and oncogenesis of gastric cancer stem cells." J Cell Mol Med. 2021 Jul;25(14):6584-6601. PMID: 34075691
- 7. Yang Fu, Runqing Huang, et al. "LncRNA ENSG00000254615 Modulates Proliferation and 5-FU Resistance by Regulating p21 and Cyclin D1 in Colorectal Cancer." Cancer Invest. 2021 Oct;39(9):696-710. PMID: 33938344
- 8. Yan L, Ding B, et al. "Inhibition of SMYD2 suppresses tumor progression by down-regulating microRNA-125b and attenuates multi-drug resistance in renal cell carcinoma." Theranostics. 2019 Oct 22;9 (26):8377-8391. PMID: 31754403
- 9. Feng M, Jin JQ, et al. "Pharmacological inhibition of β-catenin/BCL9 interaction overcomes resistance to immune checkpoint blockades by modulating T(reg) cells." Sci Adv. 2019 May 8;5(5):eaau5240. PMID: 31086813
- 10. Cho SY, Chae J, et al. "Unstable Genome and Transcriptome Dynamics during Tumor Metastasis Contribute to Therapeutic Heterogeneity in Colorectal Cancers." Clin Cancer Res. 2019 Jan 22. PMID: 30670495
- 11. Zhang B, Cui B, et al. "ATR activated by EB virus facilitates chemotherapy resistance to cisplatin or 5-fluorouracil in human nasopharyngeal carcinoma." Cancer Manag Res. 2019 Jan 9;11:573-585. PMID: 30666155
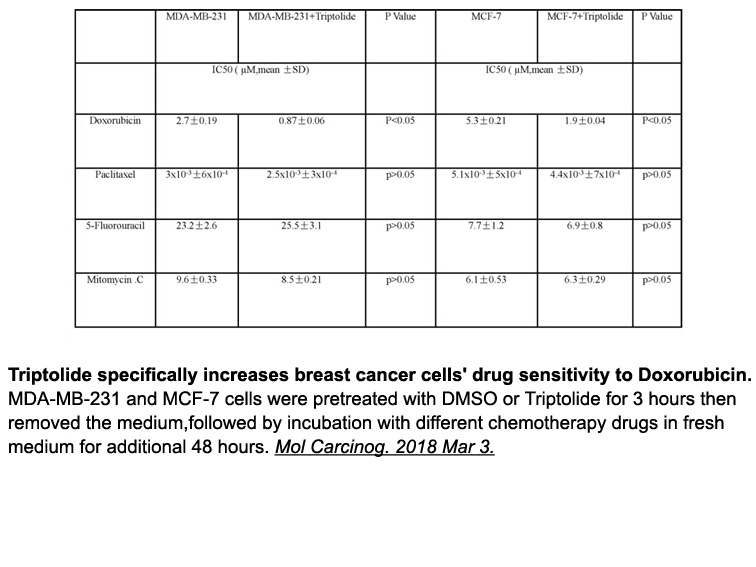
- 12. Deng Y, Li F, et al. "Triptolide sensitizes breast cancer cells to Doxorubicin through the DNA damage response inhibition." Mol Carcinog. 2018 Jun;57(6):807-814. PMID: 29500880
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 130.1 |
| Cas No. | 51-21-8 |
| Formula | C4H3FN2O2 |
| Synonyms | 5-Fluorouracil,5-FU, Efudex, Adrucil, Carac |
| Solubility | insoluble in EtOH; ≥10.04 mg/mL in H2O with gentle warming and ultrasonic; ≥13.04 mg/mL in DMSO |
| Chemical Name | 5-fluoro-1H-pyrimidine-2,4-dione |
| SDF | Download SDF |
| Canonical SMILES | O=C(C(F)=CN1)NC1=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Human colon carcinoma cell line HT-29 |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
0.01 ~ 10 μM; 7 days |
|
Applications |
In human colon carcinoma cell line HT-29, Fluorouracil suppressed cell viability, with the IC50 value of 2.5 μM. |
| Animal experiment [2]: | |
|
Animal models |
C56B1/6 mice bearing Colon 38 tumor |
|
Dosage form |
100 mg/kg; i.p.; weekly |
|
Applications |
Fluorouracil (100mg/kg) significantly suppressed tumor growth of murine colon carcinoma Colon 38 with tumor-doubling time (TD), growth-delay factor (GDF), and T/C of 26.5 days, 4.4, and 14%. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Schwartz EL, Baptiste N, Wadler S, Makower D. Thymidine phosphorylase mediates the sensitivity of human colon carcinoma cells to 5-fluorouracil. J Biol Chem. 1995 Aug 11;270(32):19073-7. [2]. Van Laar JA, Rustum YM, Van der Wilt CL, Smid K, Kuiper CM, Pinedo HM, Peters GJ. Tumor size and origin determine the antitumor activity of cisplatin or 5-fluorouracil and its modulation by leucovorin in murine colon carcinomas. Cancer Chemother Pharmacol. 1996;39(1-2):79-89. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
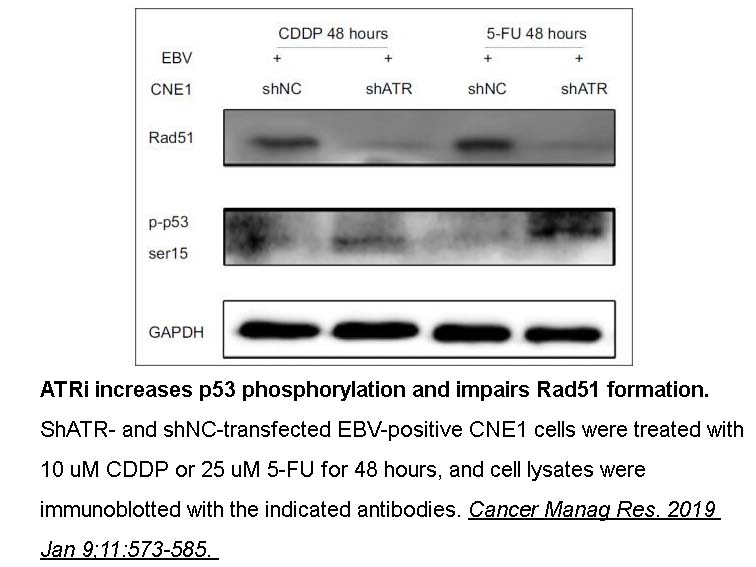
Related Biological Data
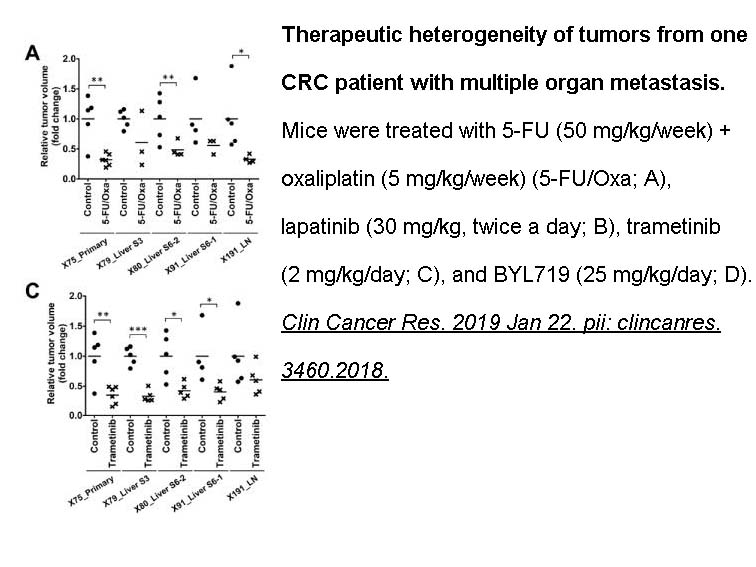
Related Biological Data
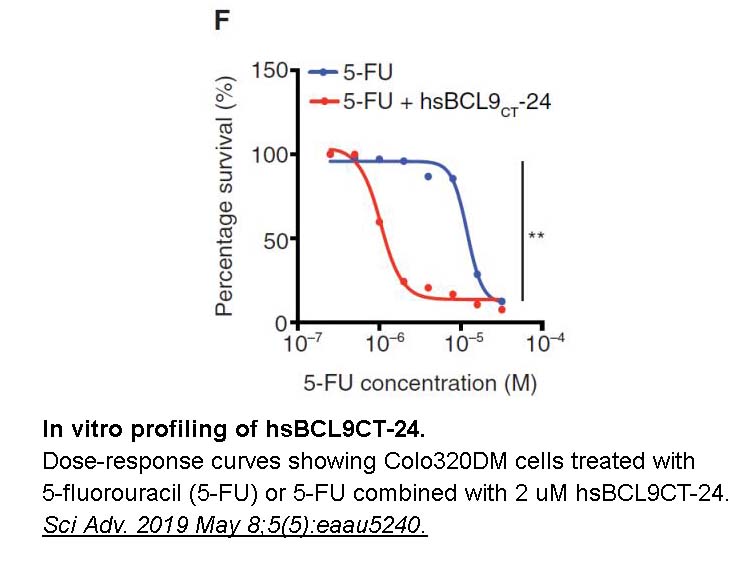
Related Biological Data
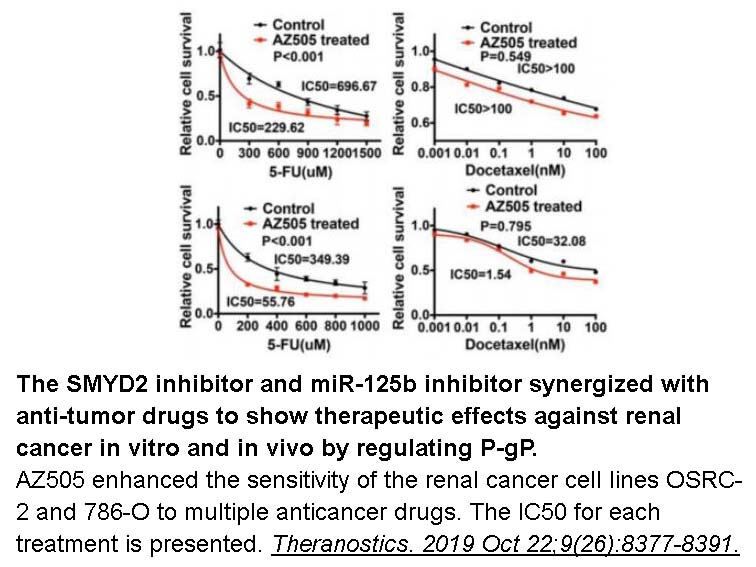
Related Biological Data
Related Biological Data