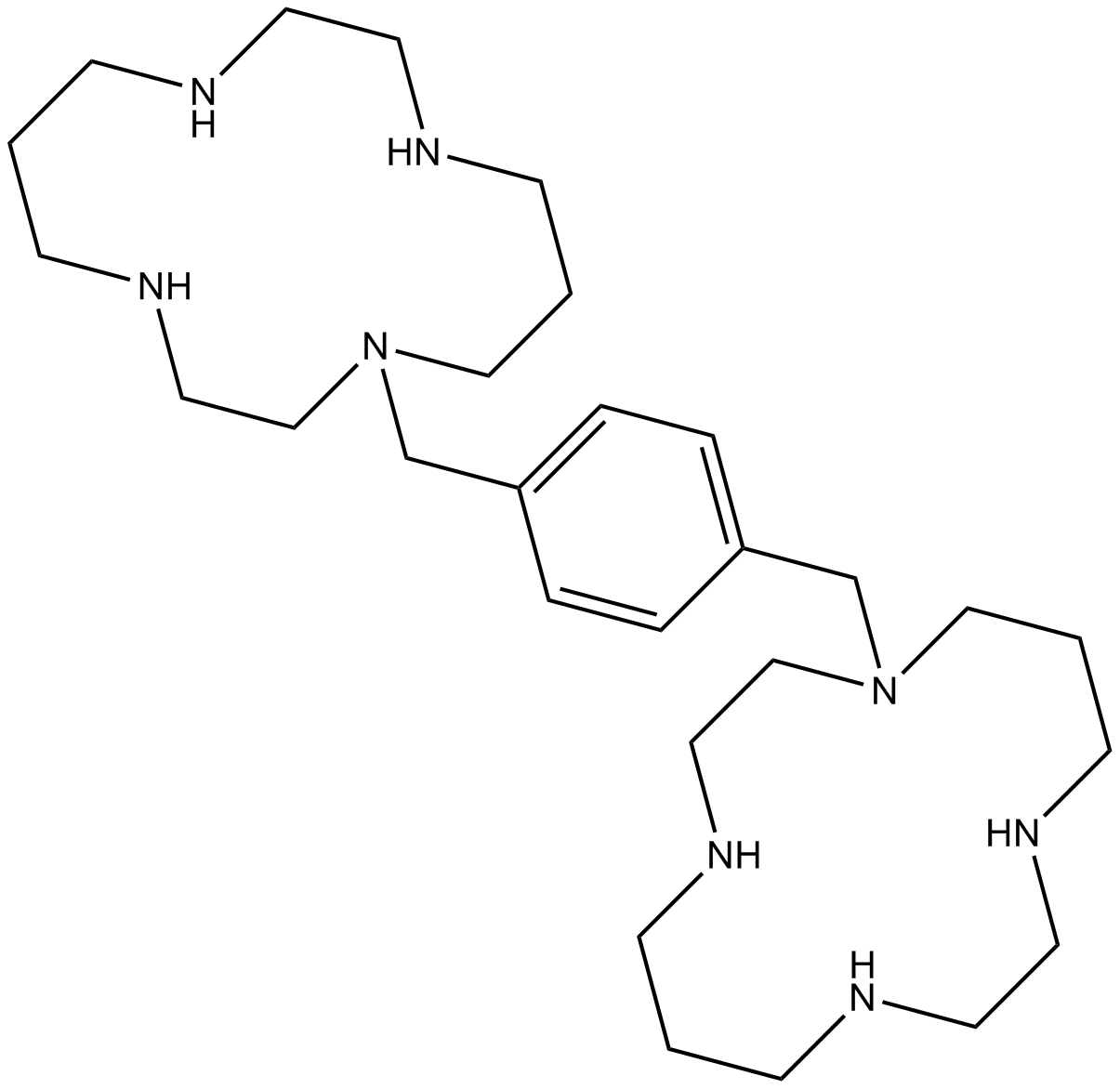
Plerixafor (AMD3100)
Plerixafor (AMD3100) is a small-molecule antagonist of CXCR4 and CXCL12-mediated chemotaxis with IC50 of 44 nM and 5.7 nM, respectively [1].
CXCR4 and SDF-1 are key factors in regulating cancer cell invasion and metastasis, and plerixafor can prevent the binding of SDF-1 to CXCR4 for inhibiting cancer metastasis [2]. Plerixafor interfered with CXCL12/CXCR4 mediated retention of hematopoietic stem cells in the bone marrow, and resulted in their mobilization to the blood [3]. Plerixafor amplified the release of circulating neutrophils from the oriented area in the lung, while simultaneously preventing neutrophil returned to the bone marrow [4]. Three adults with WHIM syndrome were subcutaneously injected 0.01 to 0.02 mg/kg plerixafor twice daily for 6 months, circulating leukocytes were constantly increased, and associated with fewer infections [5].
References:
[1]Zabel BA, Wang Y, Lewén S, Berahovich RD, Penfold ME, Zhang P, Powers J, Summers BC, Miao Z, Zhao B, Jalili A, Janowska-Wieczorek A, Jaen JC, Schall TJ. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009 Sep 1;183(5):3204-11.
[2].Li J, Oupický D. Effect of biodegradability on CXCR4 antagonism, transfection efficacy and antimetastatic activity of polymeric Plerixafor.Biomaterials. 2014 Jul;35(21):5572-9.
[3]. Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008 Jan;15(1):49-58.
[4]. Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JL, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JK, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013 Oct 21;210(11):2321-36.
[5]. McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DA, Merideth MA, Giuntoli RL, Evbuomwan MO, Littel P, Marquesen MM, Hilligoss D, DeCastro R, Grimes GJ, Hwang ST, Pittaluga S, Calvo KR, Stratton P, Cowen EW, Kuhns DB, Malech HL, Murphy PM. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014 Apr 10;123(15):2308-16.
- 1. Sapir Levin, Madeleine Benguigui, et al. "Immature monocytic cells within tumors differentiate into immunosuppressive cells in resistant tumors to immunotherapy." iScience Volume 28, Issue 8113141 August 15, 2025
- 2. Rotem Menachem, Igor Nudelman, et al. "Bone Marrow-TargetedLiposomes Loaded with BortezomibOvercome Multiple Myeloma Resistance." ACS Nano. 2025 Apr 1;19(12):11684-11701. PMID: 40117329
- 3. Hossein Khorramdelazad, Kowsar Bagherzadeh, et al. "A1, an innovative fluorinated CXCR4 inhibitor, redefines the therapeutic landscape in colorectal cancer." Cancer Cell Int. 2025 Jan 5;25(1):5. PMID: 39757159
- 4. Xizhao Chen, Tiantian Wang, et al. "Cross-species single-cell analysis uncovers the immunopathological mechanisms associated with IgA nephropathy progression." JCI Insight. 2024 May 8;9(9):e173651. PMID: 38716725
- 5. Qisheng Xiong, Ningze Zhang, et al. "Engineer a pre‐metastatic niched microenvironment to attract breast cancer cells by utilizing a 3D printed polycaprolactone/nano‐hydroxyapatite osteogenic scaffold - An in vitro model system for proof of concept." J Biomed Mater Res B Appl Biomater. 2022 Feb 3. PMID: 35112785
- 6. Shuang Zou, Dongchen Zhang, et al. "JMJD3 promotes the epithelial-mesenchymal transition and migration of glioma cells via the CXCL12/CXCR4 axis." Oncology Letters. October 9, 2019.
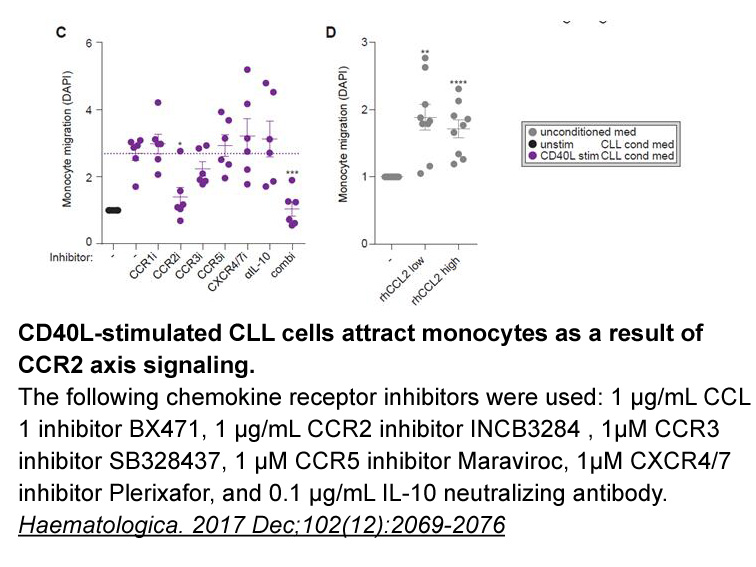
- 7. van Attekum MHA, van Bruggen JAC, et al. "CD40 signaling instructs chronic lymphocytic leukemia cells to attract monocytes via the CCR2 axis." Haematologica. 2017 Dec;102(12):2069-2076. PMID:28971904
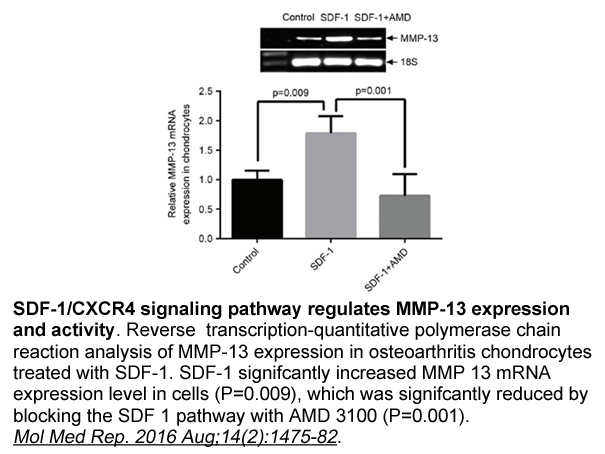
- 8. Li P, Deng J, et al."Blockade of hypoxia-induced CXCR4 with AMD3100 inhibits production of OA-associated catabolic mediators IL-1β and MMP-13." Mol Med Rep. 2016 Aug;14(2):1475-82. PMID:27356492
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 502.78 |
| Cas No. | 110078-46-1 |
| Formula | C28H54N8 |
| Solubility | ≥25.14 mg/mL in EtOH; insoluble in DMSO; ≥2.9 mg/mL in H2O with gentle warming |
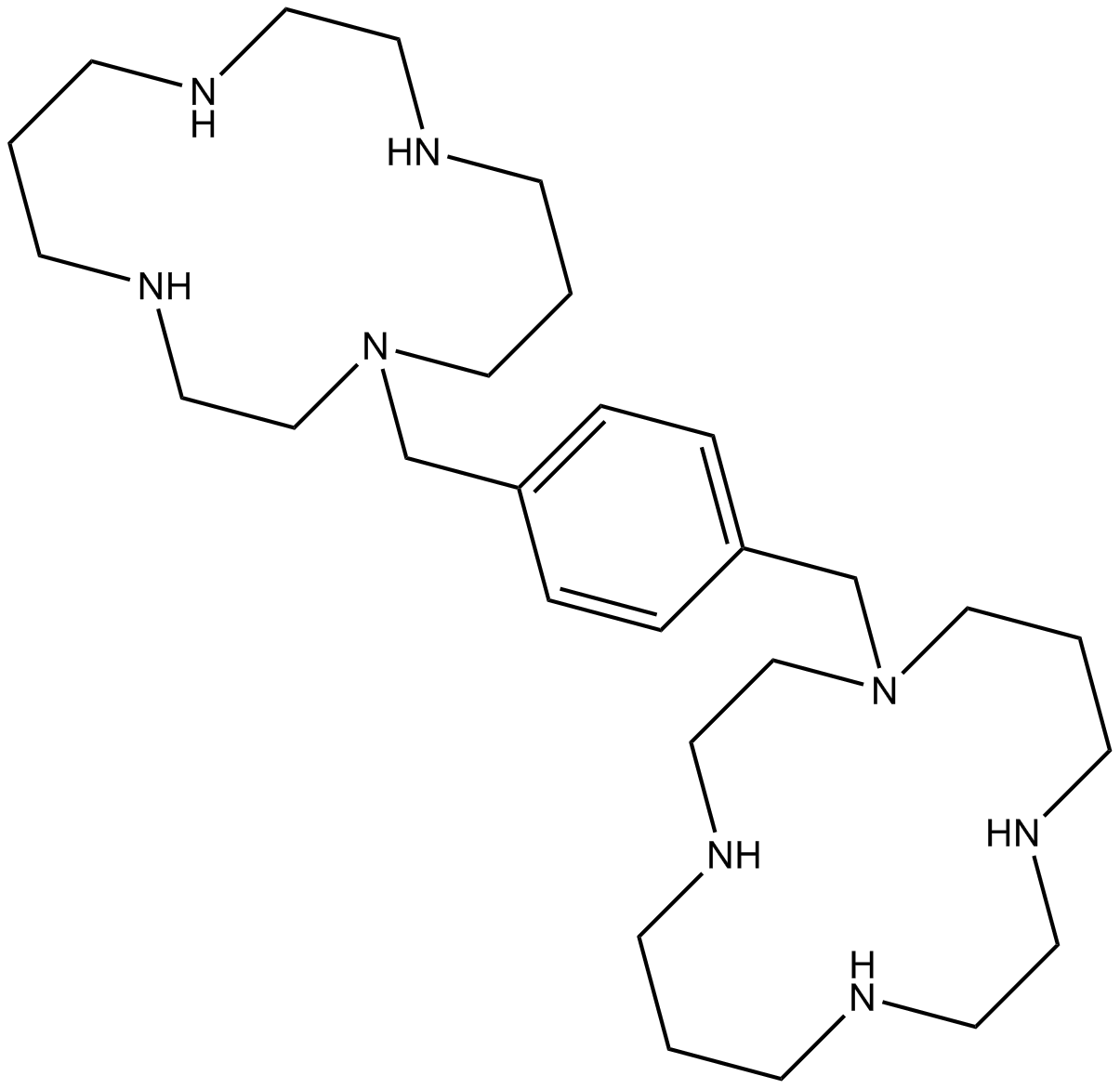
| Chemical Name | 1-[[4-(1,4,8,11-tetrazacyclotetradec-1-ylmethyl)phenyl]methyl]-1,4,8,11-tetrazacyclotetradecane |
| SDF | Download SDF |
| Canonical SMILES | C1CNCCNCCCN(CCNC1)CC2=CC=C(C=C2)CN3CCCNCCNCCCNCC3 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Kinase experiment [1]: | |
|
Receptor binding assays |
For the competition binding studies against CXCR4, a concentration range of Plerixafor was incubated for 3 hours at 4°C in binding buffer (PBS containing 5 mM MgCl2, 1 mM CaCl2, 0.25% BSA, pH 7.4) with 5 × 105 CCRF-CEM cells and 100 pM 125I-SDF-1α (2200 Ci/mmol) in Milipore DuraporeTM filter plates. Unbound 125I-SDF-1α was removed by washing with cold 50 mM HEPES, 0.5 M NaCl pH 7.4. The competition binding assay against BLT1 was performed on membranes from CHO-S cells expressing recombinant BLT1. The membranes were prepared by mechanical cell lysis followed by high speed centrifugation, re-suspended in 50 mm HEPES, 5 mM MgCl2 buffer and flash frozen. The membrane preparation was incubated with Plerixafor for 1 hour at room temperature in an assay mixture containing 50 mM Tris, pH 7.4, 10 mM MgCl2, 10 mM CaCl2, 4 nM LTB4 mixed with 1 nM 3H-LTB4 (195.0 Ci/mmol) and 8 μg membrane. The unbound 3H-LTB4 is separated by filtration on Millipore Type GF-C filter plates. |
| Cell experiment [2]: | |
|
Cell lines |
U2OS cells expressing EGFP-CXCR4 |
|
Preparation method |
Limited solubility in DMSO. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reaction Conditions |
2.5 mg/mL; 30 min |
|
Applications |
CXCR4 and SDF-1 were key factors in regulating cancer cell invasion and metastasis, and Plerixafor effectively prevented the binding of SDF-1 to CXCR4, inhibiting cancer metastasis. |
| Animal experiment [3]: | |
|
Animal models |
C57BL/6 mice with segmental bone defect |
|
Dosage form |
5 mg/kg; i.p. |
|
Applications |
Cohorts of mice were administered with PBS, IGF1, PDGF, SCF or VEGF for five consecutive days and Plerixafor on the 5th day. The number and size of the colonies were highest in mice injected with IGF1 and Plerixafor than those treated with PDGF, SCF or VEGF plus Plerixafor. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Fricker SP, Anastassov V, Cox J, Darkes MC, Grujic O, Idzan SR, Labrecque J, Lau G, Mosi RM, Nelson KL, Qin L, Santucci Z, Wong RS. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006 Aug 28;72(5):588-96. [2]. Li J, Oupick? D. Effect of biodegradability on CXCR4 antagonism, transfection efficacy and antimetastatic activity of polymeric Plerixafor.Biomaterials. 2014 Jul;35(21):5572-9. [3]. Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012 Apr;50(4):1012-8. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data